Cyclin-dependent kinase 4 drives cystic kidney disease in the absence of mTORC1 signaling activity
IF 14.8
1区 医学
Q1 UROLOGY & NEPHROLOGY
引用次数: 0
Abstract
Progression of cystic kidney disease has been linked to activation of the mTORC1 signaling pathway. Yet the utility of mTORC1 inhibitors to treat patients with polycystic kidney disease remains controversial despite promising preclinical data. To define the cell intrinsic role of mTORC1 for cyst development, the mTORC1 subunit gene Raptor was selectively inactivated in kidney tubular cells lacking cilia due to simultaneous deletion of the kinesin family member gene Kif3A. In contrast to a rapid onset of cyst formation and kidney failure in mice with defective ciliogenesis, both kidney function, cyst formation discerned by magnetic resonance imaging and overall survival were strikingly improved in mice additionally lacking Raptor. However, these mice eventually succumbed to cystic kidney disease despite mTORC1 inactivation. In-depth transcriptome analysis revealed the rapid activation of other growth-promoting signaling pathways, overriding the effects of mTORC1 deletion and identified cyclin-dependent kinase (CDK) 4 as an alternate driver of cyst growth. Additional inhibition of CDK4-dependent signaling by the CDK4/6 inhibitor Palbociclib markedly slowed disease progression in mice and human organoid models of polycystic kidney disease and potentiated the effects of mTORC1 deletion/inhibition. Our findings indicate that cystic kidneys rapidly adopt bypass mechanisms typically observed in drug resistant cancers. Thus, future clinical trials need to consider combinatorial or sequential therapies to improve therapeutic efficacy in patients with cystic kidney disease.
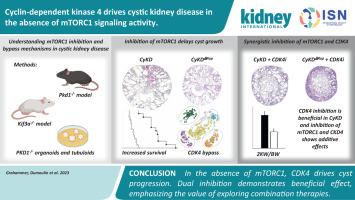
在缺乏 mTORC1 信号活动的情况下,细胞周期蛋白依赖性激酶 4 可驱动囊性肾病。
囊性肾病的进展与 mTORC1 信号通路的激活有关。然而,尽管临床前数据很有希望,mTORC1抑制剂治疗多囊肾患者的效用仍存在争议。为了确定 mTORC1 在囊肿发育过程中的细胞内在作用,研究人员选择性地使 mTORC1 亚基基因 Raptor 在因同时缺失驱动蛋白家族成员基因 Kif3A 而缺乏纤毛的肾小管细胞中失活。与纤毛发生缺陷的小鼠迅速出现囊肿形成和肾衰竭相反,额外缺乏 Raptor 的小鼠的肾功能、磁共振成像显示的囊肿形成和总体存活率都显著提高。然而,尽管mTORC1失活,这些小鼠最终还是死于囊性肾病。深入的转录组分析显示,其他促进生长的信号通路迅速激活,超过了mTORC1缺失的影响,并确定细胞周期蛋白依赖性激酶(CDK)4是囊肿生长的替代驱动因子。CDK4/6 抑制剂 Palbociclib 对 CDK4 依赖性信号传导的额外抑制明显减缓了小鼠和人类多囊肾类器官模型的疾病进展,并增强了 mTORC1 缺失/抑制的效果。我们的研究结果表明,囊性肾脏会迅速采用通常在耐药性癌症中观察到的旁路机制。因此,未来的临床试验需要考虑组合疗法或序贯疗法,以提高囊性肾病患者的疗效。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Kidney international
医学-泌尿学与肾脏学
CiteScore
23.30
自引率
3.10%
发文量
490
审稿时长
3-6 weeks
期刊介绍:
Kidney International (KI), the official journal of the International Society of Nephrology, is led by Dr. Pierre Ronco (Paris, France) and stands as one of nephrology's most cited and esteemed publications worldwide.
KI provides exceptional benefits for both readers and authors, featuring highly cited original articles, focused reviews, cutting-edge imaging techniques, and lively discussions on controversial topics.
The journal is dedicated to kidney research, serving researchers, clinical investigators, and practicing nephrologists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


