Mirtazapine in pregnancy and lactation: A systematic review of adverse outcomes
Abstract
Introduction
Peripartum depression is common and treatment with mirtazapine may be indicated. However, evidence on its safety in pregnancy and lactation is fragmented.
The objective of this systematic review was to evaluate the literature on the safety of mirtazapine in pregnancy and lactation.
Methods
PubMed, Embase, Medline, PsycInfo, and clinicaltrials.gov were searched for ‘antidepressants’ or ‘mirtazapine’ in combination with ‘pregnancy’, ‘lactation’ or ‘offspring’.
No restrictions on type of study were applied and selection was performed by two independent reviewers using Covidence. Two reviewers extracted data and performed risk of bias assessment and evidence synthesis was performed for each outcome individually.
The protocol was registered at PROSPERO (registration number CRD42021275127).
Results
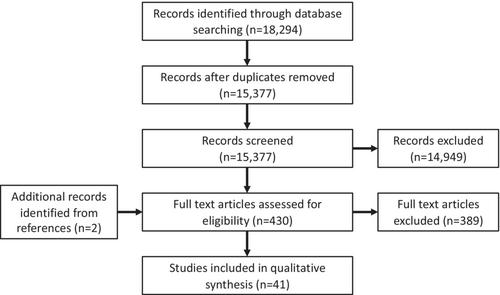
The initial search yielded 15,380 articles after removal of duplicates. After screening based on title and abstract, 431 articles remained for full text review. Of these, 41 studies were included (15 cohort studies, one case–control study, 11 case series, and 14 case reports). In most studies, the outcomes in mirtazapine-exposed pregnancies were comparable to controls. However, results on congenital malformations and spontaneous abortion were conflicting. Neonatal adaptation syndrome was reported after mirtazapine exposure in late pregnancy. Data on mirtazapine exposure during lactation were scarce.
Conclusions
We identified no substantial evidence indicating that mirtazapine exposure is associated with adverse outcomes in pregnancy or in offspring, other than neonatal adaptation syndrome. However, overall quality of evidence was low, and results on congenital malformations and spontaneous abortions were conflicting. Data on mirtazapine exposure through breastfeeding were limited and did not allow for conclusions.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


