Keggin structure heteropolyacid-catalyzed phosphinylation of secondary propargyl alcohols with phosphine oxides to γ-ketophosphine oxides†
IF 4.3
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Phosphotungstic acid with a Keggin structure as an efficient, simple and green catalyst for the phosphinylation of secondary propargyl alcohols with phosphine oxides to afford γ-ketophosphine oxides with up to 88% isolated yield was developed using dimethyl carbonate as a green solvent. Diaryl- or alkylaryl-substituted propargyl alcohols, and diaryl or arylalkylphosphine oxides could tolerate the system, which reduced the catalyst dosage, and avoided the use of multi-components and toxic solvents. More interestingly, phosphotungstic acid exhibited the best activity when 0.58 moles of water were added per mole of HPWA, elevating the yield from 55% to 85%. An 18O labelled product was afforded using trace H218O instead of H2O, indicating the participation of water in the reaction. Besides, our work underscores the importance and effect of a small amount of water, acting to promote the transformation of secondary propargyl alcohols into enones, which should be the real intermediates of the reaction. A mechanism involving a carbocation, Meyer–Schuster rearrangement and Michael addition of enones was proposed.
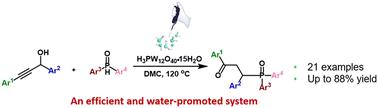
Keggin 结构杂多酸催化仲丙炔醇与膦氧化物的膦酰化反应,生成 γ-酮膦氧化物。
该研究以碳酸二甲酯为绿色溶剂,开发了一种具有 Keggin 结构的磷钨酸,作为一种高效、简单和绿色催化剂,用于仲丙炔醇与膦氧化物的膦化反应,生成γ-酮膦氧化物,分离收率高达 88%。二芳基或烷基芳基取代的丙炔醇和二芳基或芳基烷基膦氧化物可以耐受该体系,从而减少了催化剂用量,避免了多组分和有毒溶剂的使用。更有趣的是,当每摩尔 HPWA 加入 0.58 摩尔水时,磷钨酸的活性最佳,产率从 55% 提高到 85%。使用微量 H218O 而不是 H2O 可以得到 18O 标记的产物,这表明水参与了反应。此外,我们的工作还强调了少量水的重要性和作用,它能促进仲丙炔醇转化为烯酮,而烯酮应该是反应的真正中间产物。我们提出了一个涉及羰基化、Meyer-Schuster 重排和烯酮的迈克尔加成的机理。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Communications
化学-化学综合
CiteScore
8.60
自引率
4.10%
发文量
2705
审稿时长
1.4 months
期刊介绍:
ChemComm (Chemical Communications) is renowned as the fastest publisher of articles providing information on new avenues of research, drawn from all the world''s major areas of chemical research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


