Construction of arylthio/arylamino methylene bonds by addition–elimination of nitroolefins with aromatic thiols and amines†
IF 2.7
3区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
A highly efficient, catalyst-free, metal-free, atom economical green protocol for the synthesis of arylthio/arylamino methylene compounds by a Michael attack of arylthiols and anilines on nitroolefins derived from acenaphthaquinone and isatin has been developed. The method needs methanol as a reaction solvent and does not require any recrystallization, work-up process or column chromatography. (E)-Arylthio alkenes and (Z)-arylamino alkenes were obtained as the sole products. The results obtained from computational studies using density functional theory on ORCA program (B3LYP/def2-SVP level) are in good agreement with the data obtained from the single crystal X-ray analysis.
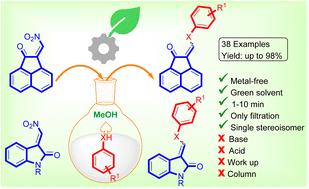
通过硝基烯烃与芳香族硫醇和胺的加成-消除作用构建芳硫基/芳氨基亚甲基键。
本研究开发了一种高效、无催化剂、无金属、原子经济的绿色方法,用于通过芳基硫醇和苯胺对源自苊醌和异atin 的硝基烯烃的迈克尔反应合成芳基硫代/芳基氨基亚甲基化合物。该方法只需甲醇作为反应溶剂,无需任何重结晶、加工过程或柱层析。(E)-芳硫基烯和(Z)-芳氨基烯被作为唯一产物获得。使用 ORCA 程序(B3LYP/def2-SVP 级)密度泛函理论进行计算研究得出的结果与单晶 X 射线分析获得的数据十分吻合。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic & Biomolecular Chemistry
化学-有机化学
CiteScore
5.50
自引率
9.40%
发文量
1056
审稿时长
1.3 months
期刊介绍:
Organic & Biomolecular Chemistry is an international journal using integrated research in chemistry-organic chemistry. Founded in 2003 by the Royal Society of Chemistry, the journal is published in Semimonthly issues and has been indexed by SCIE, a leading international database. The journal focuses on the key research and cutting-edge progress in the field of chemistry-organic chemistry, publishes and reports the research results in this field in a timely manner, and is committed to becoming a window and platform for rapid academic exchanges among peers in this field. The journal's impact factor in 2023 is 2.9, and its CiteScore is 5.5.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


