Brønsted Acid Triggers [6/7 + 1] Cascade Cyclization by N-Alkyl Amine C(sp3)-N Cleavage: Mild Synthesis of Benzo[1,4]oxazepane and Dihydrobenzo[1,5]oxazocine.
IF 3.3
2区 化学
Q1 CHEMISTRY, ORGANIC
The Journal of Organic Chemistry
Pub Date : 2024-09-20
Epub Date: 2024-09-01
DOI:10.1021/acs.joc.4c01827
引用次数: 0
Abstract
A catalyst-free mild synthesis was reported to produce medium-ring oxazepane and oxazocine derivatives from aminomaleimides and N-alkyl amines. The substrate and acidic additives were employed to cleave the C(sp3)-N bond as a one-carbon synthon for C-C and C-O coupling, thus facilitating the [n + 1] cascade cyclization reaction, which enabled the construction of seven- and eight-membered N,O-heterocycles at room temperature. The method exhibits abroad substrate scope and remarkable tolerance toward various functional groups (seven-membered 28 examples, eight-membered 8 examples, and activated N-alkyl amine 12 examples) and utilization of natural products (2 examples).
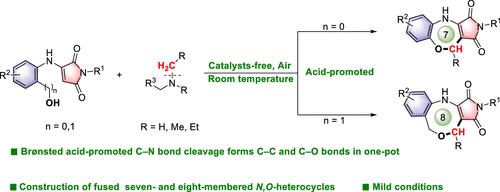
布氏酸通过 N-烷基胺 C(sp3)-N 裂解引发 [6/7 + 1] 级联环化:苯并[1,4]氧氮杂环庚烷和二氢苯并[1,5]氧氮杂环辛的温和合成。
报告采用无催化剂温和合成法,从氨基马来酰亚胺和 N-烷基胺中制备中环草氮杂环庚烷和草氮杂环辛衍生物。利用底物和酸性添加剂裂解 C(sp3)-N 键作为 C-C 和 C-O 偶联的单碳合剂,从而促进了[n + 1]级联环化反应,在室温下构建了七元和八元的 N,O-杂环。该方法的底物范围广泛,对各种官能团(七元 28 例、八元 8 例和活化 N-烷基胺 12 例)和天然产物(2 例)的利用具有显著的耐受性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

The Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
The Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


