Exploring the Substrate Scope and Catalytic Promiscuity of Nitroreductase‐Like Enzymes
IF 4.4
2区 化学
Q2 CHEMISTRY, APPLIED
引用次数: 0
Abstract
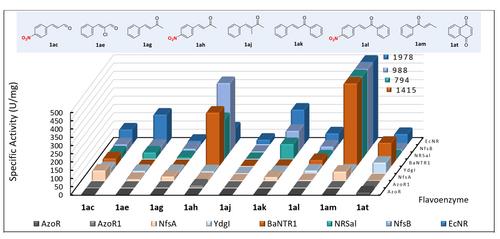
Flavin‐dependent nitroreductases are gaining attention as biocatalysts for the synthesis of pharmaceutically active compounds and their precursors. Here, we examined a panel of nitroreductase‐like flavoenzymes for their reductase activity towards a wide variety of aromatic and aliphatic nitro compounds, nitroolefins, and α,β‐unsaturated carbonyl compounds. Several of these flavoenzymes displayed high reductase activity and achieved excellent conversion of diverse nitroarenes, nitroolefins and α,β‐unsaturated carbonyl compounds, accomplishing good product yields in semi‐preparative scale reactions (up to 97%). In addition to the catalytic promiscuity of several of these flavoenzymes, being able to perform the reduction of nitro groups (nitroreductase activity) as well as C=C groups (ene‐reductase activity), this study also revealed that some flavoenzymes exhibit high chemo‐, regio‐ and/or enantioselectivity, making them attractive enzymes for use in organic synthesis.
探索类硝基还原酶的底物范围和催化杂交性
依赖黄素的硝基还原酶作为合成具有医药活性的化合物及其前体的生物催化剂,正受到越来越多的关注。在这里,我们研究了一组类似硝基还原酶的黄酶类,以检测它们对多种芳香族和脂肪族硝基化合物、硝基烯烃以及α,β-不饱和羰基化合物的还原酶活性。这些黄酮酶中有几种显示出很高的还原酶活性,对各种硝基烯烃、硝基烯烃和α,β-不饱和羰基化合物有很好的转化效果,在半制备规模的反应中获得了很好的产品收率(高达 97%)。除了其中几种黄酮酶具有催化杂交性,能够进行硝基还原(硝基还原酶活性)和 C=C 基团还原(烯还原酶活性)之外,这项研究还揭示了一些黄酮酶表现出高化学、区域和/或对映体选择性,使它们成为有机合成中极具吸引力的酶。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Advanced Synthesis & Catalysis
化学-应用化学
CiteScore
9.40
自引率
7.40%
发文量
447
审稿时长
1.8 months
期刊介绍:
Advanced Synthesis & Catalysis (ASC) is the leading primary journal in organic, organometallic, and applied chemistry.
The high impact of ASC can be attributed to the unique focus of the journal, which publishes exciting new results from academic and industrial labs on efficient, practical, and environmentally friendly organic synthesis. While homogeneous, heterogeneous, organic, and enzyme catalysis are key technologies to achieve green synthesis, significant contributions to the same goal by synthesis design, reaction techniques, flow chemistry, and continuous processing, multiphase catalysis, green solvents, catalyst immobilization, and recycling, separation science, and process development are also featured in ASC. The Aims and Scope can be found in the Notice to Authors or on the first page of the table of contents in every issue.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


