Molybdenum‐Catalyzed Intramolecular Deoxygenative Annulation of 2‐Acylazobenzenes to Access N2,C3‐Disubstituted 2H‐Indazoles
IF 4.4
2区 化学
Q2 CHEMISTRY, APPLIED
引用次数: 0
Abstract
A molybdenum‐catalyzed synthesis of N2,C3‐disubstituted 2H‐indazoles from readily available 2‐acylazobenzenes via deoxygenation of C=O and annulation has been described. The non‐noble metal catalytic system has good tolerance of functional groups, and various N2,C3‐disubstituted 2H‐indazoles have been constructed in 24% to 99% yield. This reaction is easy to scale‐up and has shown its applications in deriving valuable fluorescent and bioactive compounds. The plausible mechanism shows the plausible processes of molybdenum‐catalyzed deoxygenative annulation.
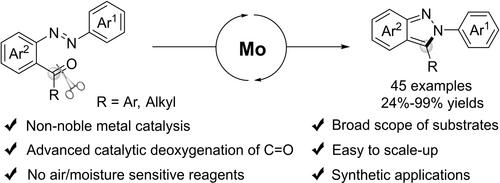
钼催化 2-酰基偶氮苯分子内脱氧合成以获得 N2、C3-二取代的 2H-indazoles
该研究描述了一种钼催化的合成方法,通过 C=O 的脱氧和环化,从容易获得的 2-acylazobenzenes 合成 N2,C3-二取代的 2H-吲唑。该非贵金属催化体系对官能团具有良好的耐受性,并以 24% 至 99% 的收率合成了各种 N2,C3-二取代的 2H-indazoles 。该反应易于放大,在衍生有价值的荧光和生物活性化合物方面已显示出其应用价值。合理的机理显示了钼催化脱氧环化的合理过程。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Advanced Synthesis & Catalysis
化学-应用化学
CiteScore
9.40
自引率
7.40%
发文量
447
审稿时长
1.8 months
期刊介绍:
Advanced Synthesis & Catalysis (ASC) is the leading primary journal in organic, organometallic, and applied chemistry.
The high impact of ASC can be attributed to the unique focus of the journal, which publishes exciting new results from academic and industrial labs on efficient, practical, and environmentally friendly organic synthesis. While homogeneous, heterogeneous, organic, and enzyme catalysis are key technologies to achieve green synthesis, significant contributions to the same goal by synthesis design, reaction techniques, flow chemistry, and continuous processing, multiphase catalysis, green solvents, catalyst immobilization, and recycling, separation science, and process development are also featured in ASC. The Aims and Scope can be found in the Notice to Authors or on the first page of the table of contents in every issue.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


