Silver Sulfide Nanocrystals and Their Photodetector Applications
IF 14
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
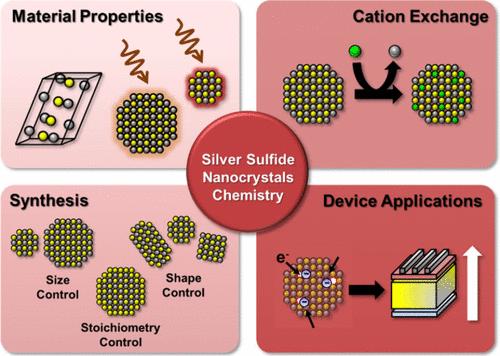
Silver sulfide nanocrystals (Ag2S NCs) exhibit unique infrared (IR) absorption and emission capabilities, drawing great interest for their broad applicability. These NCs are considered environmentally friendly alternatives to heavy metals such as lead (Pb), mercury (Hg), and cadmium (Cd) chalcogenides. This Account provides a comprehensive overview based on our research on Ag2S NCs. We investigated their synthesis over size and shape, surface stoichiometry control, postsynthetic surface composition change, and optoelectronic application. The work began with developing a synthesis protocol for the Ag2S NCs. Size-tunable and nearly monodisperse NCs were obtained through the precise control of precursor ratio. The ability to manipulate the size of the NCs enabled us to explore and adjust their optical properties. Another important aspect of the research focused on the mechanism of shape transformation. The evolution of the NCs from their initial spherical structure to more complex shapes such as rods and cubes was observed. Through rigorous investigations using a transmission electron microscope (TEM), we studied the relationship between the morphological changes and crystal facets. Investigations were also extended to surface chemistry, where methods were developed to tune the surface stoichiometry of Ag2S NCs. Perfectly stoichiometric-surfaced Ag2S NCs synthesized through ion-pair ligand-assisted surface reactions exhibited significantly increased photoluminescence (PL) and an enhanced epitaxial ZnS growth rate. Finally, we explored the cation exchange reactions of Ag2S NCs. The cation exchange reaction with indium (In) ions yielded AgInS2 NCs with size-dependent crystal structures: tetragonal for small NCs and orthorhombic for large NCs. A critical size at around 4.3 nm was observed, representing a trade-off between a thermodynamically more stable tetragonal structure and an orthorhombic structure that preserves the anionic framework. Throughout this Account, we address the challenges for the application of Ag2S NCs and propose future directions including advancements in synthesis techniques, surface chemistry, and their applications. Ag2S NCs typically show limitations such as low chemical and electrical stability, which may originate from the low lattice energy and high concentration of cation vacancies. However, such unique features can be advantageous for some applications, for example, acceptor materials in photomultiplication (PM)-type photodiodes. PM-type photodiodes were developed by combining polymeric semiconductors and Ag2S NCs. These photodiodes can amplify signals by trapping electrons within Ag2S NCs. These NCs efficiently trap multiple charge carriers from donor materials, in which their typical disadvantage is reinterpreted as a beneficial attribute for advanced device applications. In order to enhance the electron trapping efficiency, we synthesized Ag2S NCs with a cation-rich surface were synthesized. This electron trapping property resulted in an optimized PM-type photodiode with a high EQE of over 170,000% and a specific detectivity of 3 × 1013 Jones. We anticipate that this Account will provide comprehensive insights into the chemistry and optoelectronic applications of the Ag2S NCs.
硫化银纳米晶体及其光电探测器应用
硫化银纳米晶体(Ag2S NCs)具有独特的红外线(IR)吸收和发射能力,因其广泛的适用性而备受关注。这些 NCs 被认为是铅(Pb)、汞(Hg)和镉(Cd)等重金属瑀的环保替代品。本开户绑定手机领体验金全面概述了我们对 Ag2S NCs 的研究。我们研究了它们的合成尺寸和形状、表面化学计量控制、合成后表面成分变化以及光电应用。这项工作从开发 Ag2S NCs 的合成方案开始。通过精确控制前驱体比例,获得了尺寸可调且接近单分散的 NCs。操纵 NCs 大小的能力使我们能够探索和调整它们的光学特性。研究的另一个重要方面集中在形状转变的机制上。我们观察了 NCs 从最初的球形结构向更复杂形状(如棒状和立方体)的演变过程。通过使用透射电子显微镜(TEM)进行严格的调查,我们研究了形态变化与晶面之间的关系。研究还扩展到了表面化学,开发出了调整 Ag2S NCs 表面化学计量的方法。通过离子对配体辅助的表面反应合成的完美化学计量表面的 Ag2S NCs 显示出显著增强的光致发光(PL)和更高的外延 ZnS 生长速率。最后,我们探讨了 Ag2S NCs 的阳离子交换反应。与铟(In)离子的阳离子交换反应产生了 AgInS2 NCs,其晶体结构与尺寸有关:小的 NCs 为四方型,大的 NCs 为正方型。观察到临界尺寸约为 4.3 nm,这代表了热力学上更稳定的四方结构和保留阴离子框架的正交结构之间的权衡。在本开户绑定手机领体验金中,我们探讨了 Ag2S NCs 应用所面临的挑战,并提出了未来的发展方向,包括合成技术、表面化学及其应用方面的进步。Ag2S NCs 通常表现出低化学稳定性和电稳定性等局限性,这可能源于低晶格能和高浓度的阳离子空位。然而,这种独特的特性在某些应用中可能是有利的,例如,作为光倍增(PM)型光电二极管的受体材料。PM 型光电二极管是通过将聚合物半导体和 Ag2S NCs 结合在一起而开发出来的。这些光电二极管可以通过在 Ag2S NCs 中捕获电子来放大信号。这些 NC 能有效捕获来自供体材料的多个电荷载流子,从而将其典型的缺点重新诠释为先进设备应用的有利特性。为了提高电子捕获效率,我们合成了表面富含阳离子的 Ag2S NCs。这种电子捕获特性产生了一种优化的 PM 型光电二极管,其 EQE 高达 170,000% 以上,特定检测率为 3 × 1013 琼斯。我们预计该开户绑定手机领体验金将为 Ag2S NCs 的化学和光电应用提供全面的见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


