Electrocatalytic reductive deuteration of arenes and heteroarenes
IF 50.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
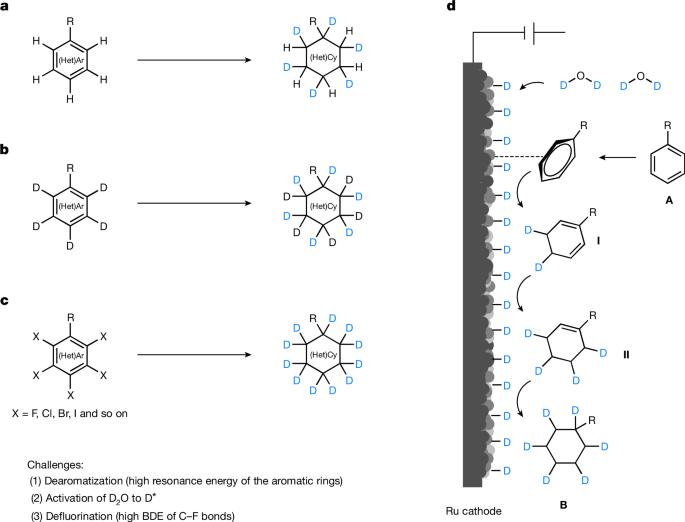
The incorporation of deuterium in organic molecules has widespread applications in medicinal chemistry and materials science1,2. For example, the deuterated drugs austedo3, donafenib4 and sotyktu5 have been recently approved. There are various methods for the synthesis of deuterated compounds with high deuterium incorporation6. However, the reductive deuteration of aromatic hydrocarbons—ubiquitous chemical feedstocks—to saturated cyclic compounds has rarely been achieved. Here we describe a scalable and general electrocatalytic method for the reductive deuteration and deuterodefluorination of (hetero)arenes using a prepared nitrogen-doped electrode and deuterium oxide (D2O), giving perdeuterated and saturated deuterocarbon products. This protocol has been successfully applied to the synthesis of 13 highly deuterated drug molecules. Mechanistic investigations suggest that the ruthenium–deuterium species, generated by electrolysis of D2O in the presence of a nitrogen-doped ruthenium electrode, are key intermediates that directly reduce aromatic compounds. This quick and cost-effective methodology for the preparation of highly deuterium-labelled saturated (hetero)cyclic compounds could be applied in drug development and metabolism studies. Reductive deuteration and deuterodefluorination of arenes and heteroarenes is achieved through a scalable and general electrocatalytic method using a nitrogen-doped ruthenium electrode and heavy water.
炔和杂环烯的电催化还原脱氘反应
在有机分子中加入氘在药物化学和材料科学领域有着广泛的应用1,2。例如,氘代药物 Austedo3、Donafenib4 和 Sotyktu5 最近已获得批准。合成高氘代化合物的方法有很多种6 。然而,将芳香烃(无处不在的化学原料)还原氘化成饱和环状化合物的方法还很少实现。在此,我们介绍一种可扩展的通用电催化方法,利用制备的掺氮电极和 D2O 对(杂)烷进行还原氘化和氘代氟化,从而得到过氘代和饱和的氘代碳产物。该方案已成功应用于 13 种高氘代药物分子的合成。机理研究表明,在掺氮 Ru 电极存在下电解 D2O 生成的 Ru-D 物种是直接还原芳香族化合物的关键中间产物。这种快速、经济地制备高 D 标记饱和(杂)循环化合物的方法可用于药物开发和代谢研究。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature
综合性期刊-综合性期刊
CiteScore
90.00
自引率
1.20%
发文量
3652
审稿时长
3 months
期刊介绍:
Nature is a prestigious international journal that publishes peer-reviewed research in various scientific and technological fields. The selection of articles is based on criteria such as originality, importance, interdisciplinary relevance, timeliness, accessibility, elegance, and surprising conclusions. In addition to showcasing significant scientific advances, Nature delivers rapid, authoritative, insightful news, and interpretation of current and upcoming trends impacting science, scientists, and the broader public. The journal serves a dual purpose: firstly, to promptly share noteworthy scientific advances and foster discussions among scientists, and secondly, to ensure the swift dissemination of scientific results globally, emphasizing their significance for knowledge, culture, and daily life.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


