Investigation of CO2 Rapid Phase Change Absorbent System Based on Amino Acid Salts and Ether Solvents
IF 3.9
3区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
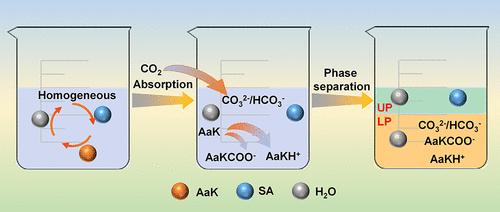
The chemical absorption method using MEA has been one of the most effective CO2 capture technologies, but the problems of low process efficiency and poor economics limit its application. In this work, the rapid phase change absorbent composed of alcohol ether solvent and potassium amino acid salt (AaK) solution was investigated, and the effects of different AaKs and ethers on the phase change behavior, phase separation mechanism, and CO2 absorption performance of the absorbent were analyzed. The results showed that when the carbon chain of the linear AaK was longer, the solubility in water was larger, and the lower liquid phase (LP) volume, phase separation time, AaK enrichment in the LP, and CO2 molar loading were larger. When the alkyl group of the branched-chain AaK was larger, the solubility in water was smaller, and the gas–liquid mass transfer, enrichment effect, and absorption rate were smaller. When the ether bonds of ether solvent were greater, the solubility of organic salts and competition for water were stronger; thus, the LP volume was smaller. Moreover, the absorption rate and CO2 loading of AaK systems were affected by the spatial structure effect of amino and carboxyl groups and the viscosity effect of solvent before absorption.
基于氨基酸盐和醚溶剂的二氧化碳快速相变吸收剂系统的研究
使用 MEA 的化学吸收法一直是最有效的二氧化碳捕集技术之一,但其工艺效率低、经济性差等问题限制了其应用。本文研究了由醇醚溶剂和氨基酸钾盐(AaK)溶液组成的快速相变吸收剂,分析了不同AaK和醇醚对吸收剂相变行为、相分离机理和二氧化碳吸收性能的影响。结果表明,当线性 AaK 的碳链较长时,其在水中的溶解度较大,下液相(LP)体积、相分离时间、LP 中 AaK 富集度和 CO2 摩尔负荷均较大。支链 AaK 的烷基越大,在水中的溶解度越小,气液传质、富集效应和吸收率也越小。当醚溶剂的醚键较大时,有机盐的溶解度和对水的竞争性较强,因此 LP 容量较小。此外,氨基和羧基的空间结构效应以及吸收前溶剂的粘度效应也影响了 AaK 体系的吸收率和二氧化碳负载量。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Industrial & Engineering Chemistry Research
工程技术-工程:化工
CiteScore
7.40
自引率
7.10%
发文量
1467
审稿时长
2.8 months
期刊介绍:
ndustrial & Engineering Chemistry, with variations in title and format, has been published since 1909 by the American Chemical Society. Industrial & Engineering Chemistry Research is a weekly publication that reports industrial and academic research in the broad fields of applied chemistry and chemical engineering with special focus on fundamentals, processes, and products.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


