Absence of a link between stabilized charge-separated state and structural changes proposed from crystal structures of a photosynthetic reaction center
IF 5.9
2区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
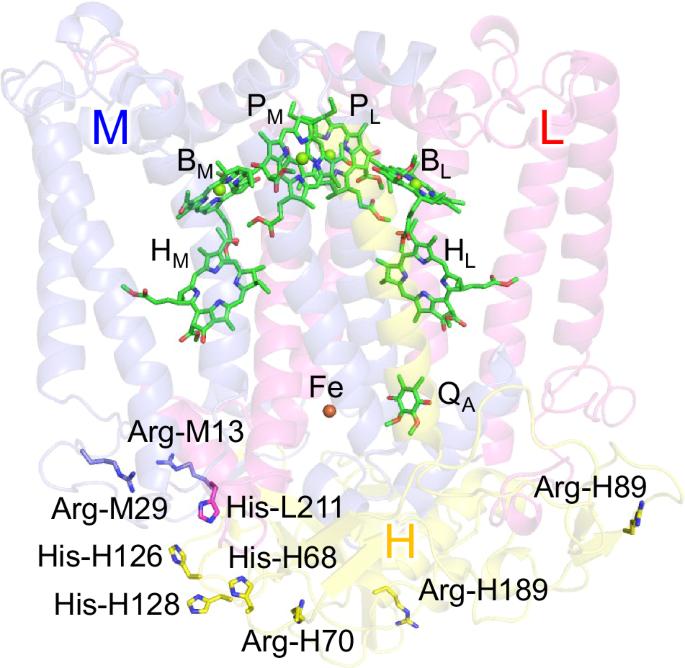
Structural differences between illuminated and unilluminated crystal structures led to the proposal that the charge-separated state was stabilized by structural changes in its membrane extrinsic protein subunit H in a bacterial photosynthetic reaction center [Katona, G. et al. Nat. Struct. Mol. Biol. 2005, 12, 630–631]. Here, we explored the proposal by titrating all titratable sites and calculating the redox potential (Em) values in these crystal structures. Contrary to the expected charge-separated states, Em for quinone, Em(QA/QA•–), is even lower in the proposed charge-separated structure than in the ground-state structure. The subunit-H residues, which were proposed to exhibit electron-density changes in the two crystal structures, contribute to an Em(QA/QA•–) difference of only <0.5 mV. Furthermore, the protonation states of the titratable residues in the entire reaction center are practically identical in the two structures. These findings indicate that the proposed structural differences are irrelevant to explaining the significant prolongation of the charge-separated-state lifetime. In bacterial photosynthetic reactions, charge-separated states are thought to be stabilized by structural changes in the membrane extrinsic protein subunit H. Here, the authors probe all titratable sites and calculate values of cofactors, and find that the redox potential for quinone is lower in the proposed charge-separated than in the ground-state structure and that the subunit-H residues contribute to a redox potential difference below 0.5 mV.
根据光合作用反应中心晶体结构提出的稳定电荷分离状态与结构变化之间缺乏联系
发光晶体结构与非发光晶体结构之间的结构差异促使人们提出,电荷分离状态是通过细菌光合作用反应中心膜外蛋白亚基 H 的结构变化来稳定的[Katona, G. et al. Nat. Struct.]在这里,我们通过滴定所有可滴定位点并计算这些晶体结构中的氧化还原电位(Em)值来探索这一提议。与预期的电荷分离状态相反,在拟议的电荷分离结构中,醌的氧化还原电位(Em(QA/QA--))甚至低于基态结构。亚单位-H残基被认为在两种晶体结构中显示出电子密度的变化,但其导致的Em(QA/QA--)差异仅为0.5 mV。此外,整个反应中心中可滴定残基的质子化状态在两种结构中几乎完全相同。这些发现表明,所提出的结构差异与解释电荷分离态寿命的显著延长无关。在细菌光合作用反应中,电荷分离态被认为是通过膜外蛋白亚基 H 的结构变化来稳定的。在这里,作者探测了所有可滴定位点并计算了辅助因子的值,结果发现,在拟议的电荷分离态结构中,醌的氧化还原电位低于基态结构,亚基 H 残基导致氧化还原电位差低于 0.5 mV。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Communications Chemistry
Chemistry-General Chemistry
CiteScore
7.70
自引率
1.70%
发文量
146
审稿时长
13 weeks
期刊介绍:
Communications Chemistry is an open access journal from Nature Research publishing high-quality research, reviews and commentary in all areas of the chemical sciences. Research papers published by the journal represent significant advances bringing new chemical insight to a specialized area of research. We also aim to provide a community forum for issues of importance to all chemists, regardless of sub-discipline.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


