Chidamide plus R-GDP for relapsed/refractory diffuse large B-cell lymphoma in patients ineligible for autologous transplantation: A prospective, single-arm, phase II study
Abstract
Background
In relapsed/refractory (R/R) diffuse large B-cell lymphoma (DLBCL), a negative prognosis is frequently linked to heightened epigenetic heterogeneity. Chidamide, a selective histone deacetylase inhibitor, shows promise as a targeted therapy for R/R DLBCL by targeting abnormal epigenetic changes associated with poor prognosis.
Methods
A cohort of 27 ineligible patients with R/R DLBCL participated in an open — label, single — arm study. Chidamide was administered orally at a dose of 30 mg twice weekly for one week during the induction monotherapy phase. The subsequent combination therapy phase involved oral chidamide at a dose of 20 mg twice weekly for two weeks, followed by a one-week discontinuation period, in conjunction with intravenous R-GDP every 21 days.
Results
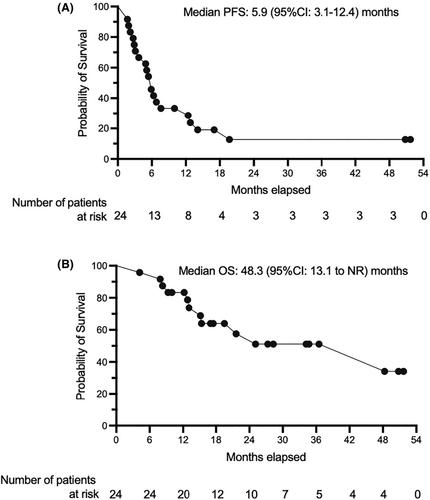
Among the cohort of 31 patients who underwent screening (median age: 67 years), 27 were ultimately included in the study, with 14 individuals successfully completing six cycles of C-R-GDP treatment. The overall best objective response rate was determined to be 79.1% (95% CI: 75.1%–83.3%), comprising a complete response rate of 45.8% (95% CI: 41.6%–49.9%) and a partial response rate of 33.3% (95% CI: 29.3%–37.4%). Within the subgroup of 14 patients who completed the full treatment regimen, the best objective response rate reached 100%, with 71.4% achieving complete response (n = 10) and 28.6% achieving partial response (n = 4). The median follow-up period for these patients was 17.0 months, ranging from 3.5 to 55 months. Progression-free survival was 5.9 months and overall survival was 48.3 months. Anemia was the most common adverse event, affecting all patients. Thrombocytopenia led to treatment interruption or dose reduction in 13 patients. Other common adverse events included hypocalcemia, hyponatremia, and hypokalemia. Three patients experienced grade 3 pneumonitis and one had grade 3 skin rash.
Conclusions
Chidamide combined with R-GDP is a safe and effective treatment option for patients with R/R DLBCL who are not eligible for autologous stem cell transplantation.
| 公司名称 | 产品信息 | 采购帮参考价格 |
|---|
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


