Cost-effectiveness analysis of osimertinib plus chemotherapy for patients with EGFR-mutated advanced non-small cell lung cancer
Abstract
Introduction
First-line osimertinib plus chemotherapy significantly prolonged progression-free survival of patients with EGFR-mutated advanced non-small cell lung cancer (NSCLC) compared to osimertinib, according to the FLAURA2 trial.
Methods
We established a Markov model to compare the cost-effectiveness of osimertinib plus chemotherapy with that of osimertinib alone. Clinical data were obtained from the FLAURA and FLAURA2 trials, and additional data were extracted from online resources and publications. Sensitivity analyses were conducted to evaluate the robustness of the findings. We used A willingness-to-pay threshold of $150,000 per quality-adjusted life-years (QALYs) gained. The main outcomes were QALYs, overall costs, incremental cost-effectiveness ratio (ICER), incremental net monetary benefit, and incremental net health benefit. Subgroup analyses were conducted according to patients' mutation type and central nervous system (CNS) metastatic status.
Results
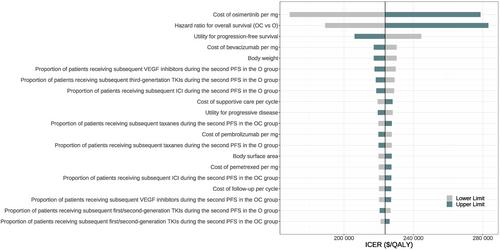
In a 20-year time horizon, the ICER of osimertinib plus chemotherapy versus osimertinib alone was $223,727.1 per QALY gained. The sensitivity analyses identified the cost of osimertinib and the hazard ratio for overall survival as the top 2 influential factors and a 1.9% probability of osimertinib plus chemotherapy to be cost-effective. The subgroup analyses revealed ICERs of $132,614.1, $224,449.8, $201,464.1, and $130,159.7 per QALY gained for L858R mutations, exon 19 deletions, CNS metastases, and no CNS metastases subgroups, respectively.
Conclusions
From the perspective of the United States health care system, osimertinib plus chemotherapy is not cost-effective compared to osimertinib alone for treatment-naïve patients with EGFR-mutated advanced NSCLC, but more favorable cost-effectiveness occurs in patients with L858R mutations and patients without baseline CNS metastases.

| 公司名称 | 产品信息 | 采购帮参考价格 |
|---|
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


