Phase separation of phospho-HDAC6 drives aberrant chromatin architecture in triple-negative breast cancer
IF 23.5
1区 医学
Q1 ONCOLOGY
引用次数: 0
Abstract
How dysregulated liquid–liquid phase separation (LLPS) contributes to the oncogenesis of female triple-negative breast cancer (TNBC) remains unknown. Here we demonstrate that phosphorylated histone deacetylase 6 (phospho-HDAC6) forms LLPS condensates in the nuclei of TNBC cells that are essential for establishing aberrant chromatin architecture. The disordered N-terminal domain and phosphorylated residue of HDAC6 facilitate effective LLPS, whereas nuclear export regions exert a negative dominant effect. Through phase-separation-based screening, we identified Nexturastat A as a specific disruptor of phospho-HDAC6 condensates, which effectively suppresses tumor growth. Mechanistically, importin-β interacts with phospho-HDAC6, promoting its translocation to the nucleus, where 14-3-3θ mediates the condensate formation. Disruption of phospho-HDAC6 LLPS re-established chromatin compartments and topologically associating domain boundaries, leading to disturbed chromatin loops. The phospho-HDAC6-induced aberrant chromatin architecture affects chromatin accessibility, histone acetylation, RNA polymerase II elongation and transcriptional profiles in TNBC. This study demonstrates phospho-HDAC6 LLPS as an emerging mechanism underlying the dysregulation of chromatin architecture in TNBC. Lu et al. investigate the involvement of liquid–liquid phase separation in the progression of triple-negative breast cancer and find that phosphorylated histone deacetylase 6 forms condensates that affect chromatin accessibility and oncogenic transcriptional programs.
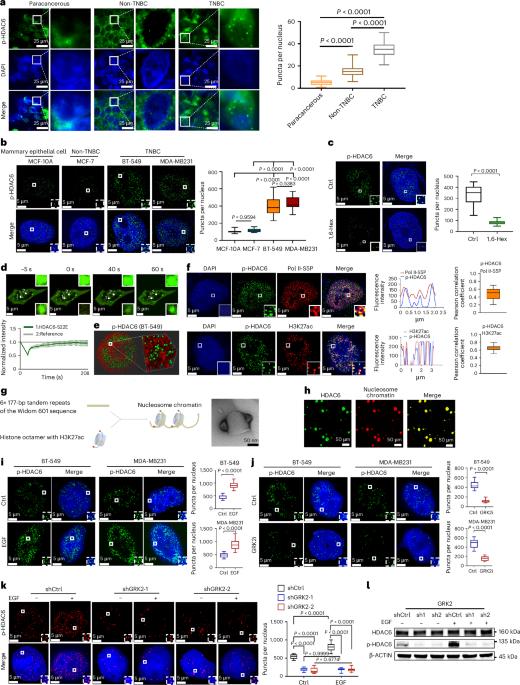
磷酸化 HDAC6 的相分离驱动三阴性乳腺癌染色质结构异常。
液-液相分离(LLPS)失调如何导致女性三阴性乳腺癌(TNBC)的肿瘤发生仍是一个未知数。在这里,我们证明了磷酸化组蛋白去乙酰化酶6(phospho-HDAC6)在TNBC细胞核中形成LLPS凝聚物,这对建立异常染色质结构至关重要。HDAC6 紊乱的 N 端结构域和磷酸化残基促进了有效的 LLPS,而核输出区域则产生了负面的主导作用。通过基于相分离的筛选,我们发现Nexturastat A是磷酸化HDAC6凝集物的特异性破坏者,它能有效抑制肿瘤生长。从机理上讲,导入素-β与磷酸化-HDAC6相互作用,促进其转位到细胞核,14-3-3θ介导凝集物的形成。磷酸-HDAC6 LLPS的破坏重建了染色质区室和拓扑关联域边界,导致染色质环路紊乱。磷酸化-HDAC6诱导的染色质结构异常会影响TNBC的染色质可及性、组蛋白乙酰化、RNA聚合酶II伸长和转录谱。这项研究表明,磷酸化 HDAC6 LLPS 是 TNBC 染色质结构失调的一种新机制。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature cancer
Medicine-Oncology
CiteScore
31.10
自引率
1.80%
发文量
129
期刊介绍:
Cancer is a devastating disease responsible for millions of deaths worldwide. However, many of these deaths could be prevented with improved prevention and treatment strategies. To achieve this, it is crucial to focus on accurate diagnosis, effective treatment methods, and understanding the socioeconomic factors that influence cancer rates.
Nature Cancer aims to serve as a unique platform for sharing the latest advancements in cancer research across various scientific fields, encompassing life sciences, physical sciences, applied sciences, and social sciences. The journal is particularly interested in fundamental research that enhances our understanding of tumor development and progression, as well as research that translates this knowledge into clinical applications through innovative diagnostic and therapeutic approaches. Additionally, Nature Cancer welcomes clinical studies that inform cancer diagnosis, treatment, and prevention, along with contributions exploring the societal impact of cancer on a global scale.
In addition to publishing original research, Nature Cancer will feature Comments, Reviews, News & Views, Features, and Correspondence that hold significant value for the diverse field of cancer research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


