Generation of rabbit single-chain variable fragments with different physicochemical and biological properties by complementary determining region-grafting technology
IF 2.3
4区 生物学
Q3 BIOTECHNOLOGY & APPLIED MICROBIOLOGY
引用次数: 0
Abstract
In this study, we have demonstrated a complementary-determining region (CDR) grafting technology for the generation of rabbit scFvs with different antigen recognition and physicochemical properties. The antigen-binding affinity of the CDR-grafted anti-CRP scFv, C1R/B1R (V1), which was generated by the CDR/framework region (CDR/FR) definition based on the traditional numbering rule, was insufficient when compared to that of the original clone, C1R, suggesting that the amino acid residues outside the original CDRs might significantly contribute to antigen recognition in rabbit scFvs. We redefined new CDRs and FRs to maintain antigen-binding affinities through the extension of multiple amino acid residues for CDRH1 and CDRH2, based on the amino acid sequence alignments of rabbit scFvs isolated from phage libraries. The new version successfully maintained the antigen binding affinity. CDR-grafted scFvs possessing a common CDR sequence and different FR sequences were successfully generated based on this new CDR/FR definition, and their physicochemical properties were further investigated. The antigen-binding activities of rabbit scFvs on Maxisorp varied between the tested clones in sandwich ELISA, supporting the idea that the combination of CDR with different FRs might change the physicochemical properties of scFvs on a solid material. The CDR-grafted scFvs possessing a frame sequence of anti-CRP scFv C2R maintained the ability to bind to protein L and were successfully purified. Expression titers showed improved solubility by diminishing the amount of insoluble scFvs. Thus, the method developed in this study is promising for generating alternatives with strict antigen binding recognition and different physicochemical properties.
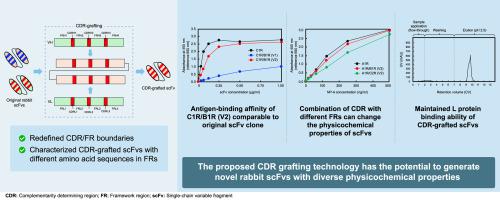
利用互补决定区接枝技术生成具有不同理化和生物特性的兔单链可变片段。
在这项研究中,我们展示了一种互补决定区(CDR)嫁接技术,用于生成具有不同抗原识别和理化性质的兔 scFv。根据传统编号规则定义CDR/框架区(CDR/FR)生成的CDR嫁接抗CRP scFv C1R/B1R(V1)与原始克隆C1R相比,抗原结合亲和力不足,这表明原始CDR外的氨基酸残基可能对兔scFv的抗原识别有重要贡献。我们根据从噬菌体文库中分离出的兔 scFvs 的氨基酸序列比对,通过延长 CDRH1 和 CDRH2 的多个氨基酸残基,重新定义了新的 CDR 和 FR,以保持抗原结合亲和力。新版本成功地保持了抗原结合亲和力。根据这一新的CDR/FR定义,成功生成了具有共同CDR序列和不同FR序列的CDR接枝scFvs,并进一步研究了它们的理化性质。在夹心酶联免疫吸附试验中,不同克隆的兔 scFvs 在 Maxisorp 上的抗原结合活性各不相同,这证明了 CDR 与不同 FR 的结合可能会改变 scFvs 在固体材料上的理化性质。具有抗 CRP scFv C2R 框架序列的 CDR 接枝 scFvs 保持了与蛋白 L 结合的能力并成功纯化。因此,本研究开发的方法有望生成具有严格抗原结合识别能力和不同理化性质的替代品。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of bioscience and bioengineering
生物-生物工程与应用微生物
CiteScore
5.90
自引率
3.60%
发文量
144
审稿时长
51 days
期刊介绍:
The Journal of Bioscience and Bioengineering is a research journal publishing original full-length research papers, reviews, and Letters to the Editor. The Journal is devoted to the advancement and dissemination of knowledge concerning fermentation technology, biochemical engineering, food technology and microbiology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


