Probing intermediate configurations of oxygen evolution catalysis across the light spectrum
IF 49.7
1区 材料科学
Q1 ENERGY & FUELS
引用次数: 0
Abstract
The oxygen evolution reaction is crucial to sustainable electro- and photo-electrochemical approaches to chemical energy production (for example, H2). Although mechanistic descriptions of the oxygen evolution reaction have been proposed, the frontier challenge is to extract the molecular details of its elementary steps. Here we discuss how advances in spectroscopy and theory are allowing for configurations of reaction intermediates to be elucidated, distinguishing between experimental approaches (static and dynamic) across a range of surface oxygen binding energies on catalysts (from ruthenium to titanium oxides). We outline how interpreting X-ray and optical spectra relies on established and newly implemented computational techniques. A key emphasis is on detecting adsorbed oxygen intermediates at the oxide/water interface by their chemical composition, electronic and vibrational levels and ion–electron kinetic pathways. Integrating the computational advances with the experimental spectra along these lines could ultimately resolve the elementary steps, elucidating how each intermediate leads to another during oxygen evolution reaction. Oxygen evolution is a critical reaction in the context of renewable fuel production via (photo)electrochemical approaches, yet our understanding of the molecular details of the reaction is limited. Here, the authors explore how specific spectroscopic probes and theory can be combined to reveal the elementary reaction steps.
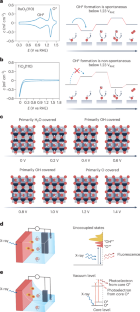
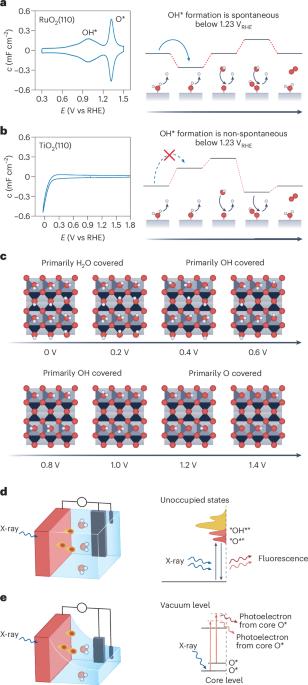
跨光谱探测氧进化催化的中间构型
氧进化反应对于以可持续的电化学和光电化学方法生产化学能(如 H2)至关重要。虽然已经提出了氧进化反应的机理描述,但前沿挑战是提取其基本步骤的分子细节。在此,我们将讨论光谱学和理论的进步如何使反应中间产物的构型得以阐明,并区分催化剂(从钌到钛氧化物)表面氧结合能范围内的实验方法(静态和动态)。我们概述了 X 射线和光学光谱的解释如何依赖于已有的和新实施的计算技术。重点是通过化学成分、电子和振动水平以及离子-电子动力学路径来检测氧化物/水界面上吸附的氧中间体。按照这些思路将计算进展与实验光谱相结合,最终可以解决基本步骤问题,阐明在氧进化反应过程中每个中间体如何导致另一个中间体。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Energy
Energy-Energy Engineering and Power Technology
CiteScore
75.10
自引率
1.10%
发文量
193
期刊介绍:
Nature Energy is a monthly, online-only journal committed to showcasing the most impactful research on energy, covering everything from its generation and distribution to the societal implications of energy technologies and policies.
With a focus on exploring all facets of the ongoing energy discourse, Nature Energy delves into topics such as energy generation, storage, distribution, management, and the societal impacts of energy technologies and policies. Emphasizing studies that push the boundaries of knowledge and contribute to the development of next-generation solutions, the journal serves as a platform for the exchange of ideas among stakeholders at the forefront of the energy sector.
Maintaining the hallmark standards of the Nature brand, Nature Energy boasts a dedicated team of professional editors, a rigorous peer-review process, meticulous copy-editing and production, rapid publication times, and editorial independence.
In addition to original research articles, Nature Energy also publishes a range of content types, including Comments, Perspectives, Reviews, News & Views, Features, and Correspondence, covering a diverse array of disciplines relevant to the field of energy.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


