A metalloenzyme platform for catalytic asymmetric radical dearomatization
IF 20.2
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Catalytic asymmetric dearomatization represents a powerful means to convert flat aromatic compounds into stereochemically well-defined three-dimensional molecular scaffolds. Using new-to-nature metalloredox biocatalysis, we describe an enzymatic strategy for catalytic asymmetric dearomatization via a challenging radical mechanism that has eluded small-molecule catalysts. Enabled by directed evolution, new-to-nature radical dearomatases P450rad1–P450rad5 facilitated asymmetric dearomatization of a broad spectrum of aromatic substrates, including indoles, pyrroles and phenols, allowing both enantioconvergent and enantiodivergent radical dearomatization reactions to be accomplished with excellent enzymatic control. Computational studies revealed the importance of additional hydrogen bonding interactions between the engineered metalloenzyme and the reactive intermediate in enhancing enzymatic activity and enantiocontrol. Furthermore, designer non-ionic surfactants were found to significantly accelerate this biotransformation, providing an alternative means to promote otherwise sluggish new-to-nature biotransformations. Together, this evolvable metalloenzyme platform opens up new avenues to advance challenging catalytic asymmetric dearomatization processes involving free radical intermediates. Catalytic asymmetric radical dearomatization has remained a daunting task due to the challenges in exerting stereocontrol over highly reactive radical intermediates. Now, using metalloredox biocatalysis, new-to-nature radical dearomatases P450rad1–P450rad5 have been engineered to facilitate asymmetric dearomatization of a broad spectrum of aromatic substrates, including indoles, pyrroles and phenols.
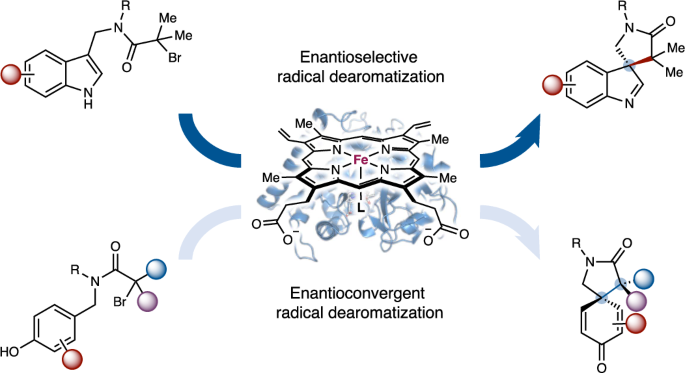
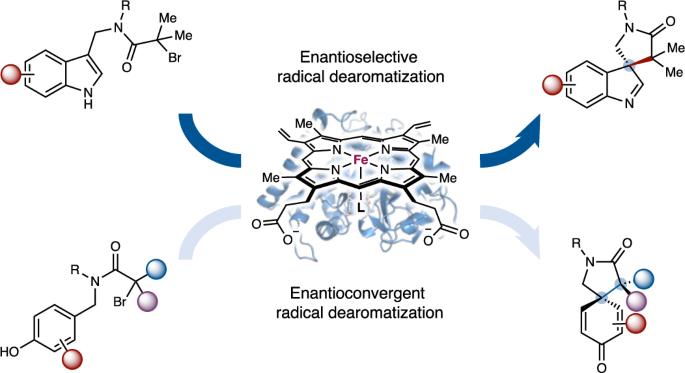
催化不对称自由基脱芳烃的金属酶平台
催化不对称脱芳烃是将平面芳香化合物转化为立体化学定义明确的三维分子支架的有力手段。利用新自然金属氧化物生物催化,我们描述了一种通过具有挑战性的自由基机制催化不对称脱芳烃的酶策略,这种机制一直为小分子催化剂所忽视。在定向进化的推动下,新自然界自由基脱芳烃酶 P450rad1-P450rad5 促进了包括吲哚、吡咯和酚在内的多种芳香底物的不对称脱芳烃反应,使对映转化和对映分歧自由基脱芳烃反应都能在出色的酶控下完成。计算研究表明,工程金属酶与反应中间体之间的额外氢键相互作用对增强酶活性和对映体控制非常重要。此外,研究还发现设计型非离子表面活性剂能显著加速这种生物转化,为促进原本缓慢的新自然生物转化提供了另一种方法。总之,这种可进化的金属酶平台为推进涉及自由基中间体的具有挑战性的催化不对称脱芳烃过程开辟了新途径。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature chemistry
化学-化学综合
CiteScore
29.60
自引率
1.40%
发文量
226
审稿时长
1.7 months
期刊介绍:
Nature Chemistry is a monthly journal that publishes groundbreaking and significant research in all areas of chemistry. It covers traditional subjects such as analytical, inorganic, organic, and physical chemistry, as well as a wide range of other topics including catalysis, computational and theoretical chemistry, and environmental chemistry.
The journal also features interdisciplinary research at the interface of chemistry with biology, materials science, nanotechnology, and physics. Manuscripts detailing such multidisciplinary work are encouraged, as long as the central theme pertains to chemistry.
Aside from primary research, Nature Chemistry publishes review articles, news and views, research highlights from other journals, commentaries, book reviews, correspondence, and analysis of the broader chemical landscape. It also addresses crucial issues related to education, funding, policy, intellectual property, and the societal impact of chemistry.
Nature Chemistry is dedicated to ensuring the highest standards of original research through a fair and rigorous review process. It offers authors maximum visibility for their papers, access to a broad readership, exceptional copy editing and production standards, rapid publication, and independence from academic societies and other vested interests.
Overall, Nature Chemistry aims to be the authoritative voice of the global chemical community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


