NaCl enhances CD8+ T cell effector functions in cancer immunotherapy
IF 27.7
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
Abstract
CD8+ T cells control tumors but inevitably become dysfunctional in the tumor microenvironment. Here, we show that sodium chloride (NaCl) counteracts T cell dysfunction to promote cancer regression. NaCl supplementation during CD8+ T cell culture induced effector differentiation, IFN-γ production and cytotoxicity while maintaining the gene networks responsible for stem-like plasticity. Accordingly, adoptive transfer of tumor-specific T cells resulted in superior anti-tumor immunity in a humanized mouse model. In mice, a high-salt diet reduced the growth of experimental tumors in a CD8+ T cell-dependent manner by inhibiting terminal differentiation and enhancing the effector potency of CD8+ T cells. Mechanistically, NaCl enhanced glutamine consumption, which was critical for transcriptional, epigenetic and functional reprogramming. In humans, CD8+ T cells undergoing antigen recognition in tumors and predicting favorable responses to checkpoint blockade immunotherapy resembled those induced by NaCl. Thus, NaCl metabolism is a regulator of CD8+ T cell effector function, with potential implications for cancer immunotherapy. Along with a back-to-back published paper from Zielisnki and co. in this issue of Nature Immunology, this paper shows that NaCl affects CD8+ T cell function by counteracting the exhaustion of these cells in the tumor microenvironment.
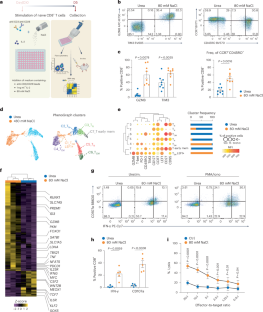

氯化钠能增强癌症免疫疗法中 CD8+ T 细胞的效应功能
CD8+ T细胞可控制肿瘤,但在肿瘤微环境中不可避免地会出现功能障碍。在这里,我们发现氯化钠(NaCl)能抵消 T 细胞的功能障碍,促进癌症消退。在 CD8+ T 细胞培养过程中补充氯化钠可诱导效应分化、IFN-γ 生成和细胞毒性,同时维持干样可塑性基因网络。因此,在人源化小鼠模型中,肿瘤特异性 T 细胞的收养性转移产生了卓越的抗肿瘤免疫力。在小鼠体内,高盐饮食通过抑制终末分化和增强 CD8+ T 细胞的效应效力,以 CD8+ T 细胞依赖的方式减少了实验性肿瘤的生长。从机理上讲,NaCl 增加了谷氨酰胺的消耗,而谷氨酰胺对于转录、表观遗传和功能重编程至关重要。在人体中,CD8+ T细胞在肿瘤中进行抗原识别并预测对检查点阻断免疫疗法的良好反应,与氯化钠诱导的CD8+ T细胞相似。因此,NaCl代谢是CD8+ T细胞效应功能的调节因子,对癌症免疫疗法具有潜在的影响。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Immunology
医学-免疫学
CiteScore
40.00
自引率
2.30%
发文量
248
审稿时长
4-8 weeks
期刊介绍:
Nature Immunology is a monthly journal that publishes the highest quality research in all areas of immunology. The editorial decisions are made by a team of full-time professional editors. The journal prioritizes work that provides translational and/or fundamental insight into the workings of the immune system. It covers a wide range of topics including innate immunity and inflammation, development, immune receptors, signaling and apoptosis, antigen presentation, gene regulation and recombination, cellular and systemic immunity, vaccines, immune tolerance, autoimmunity, tumor immunology, and microbial immunopathology. In addition to publishing significant original research, Nature Immunology also includes comments, News and Views, research highlights, matters arising from readers, and reviews of the literature. The journal serves as a major conduit of top-quality information for the immunology community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


