Intestinal organ chips for disease modelling and personalized medicine
IF 45.9
1区 医学
Q1 GASTROENTEROLOGY & HEPATOLOGY
引用次数: 0
Abstract
Alterations in intestinal structure, mechanics and physiology underlie acute and chronic intestinal conditions, many of which are influenced by dysregulation of microbiome, peristalsis, stroma or immune responses. Studying human intestinal physiology or pathophysiology is difficult in preclinical animal models because their microbiomes and immune systems differ from those of humans. Although advances in organoid culture partially overcome this challenge, intestinal organoids still lack crucial features that are necessary to study functions central to intestinal health and disease, such as digestion or fluid flow, as well as contributions from long-term effects of living microbiome, peristalsis and immune cells. Here, we review developments in organ-on-a-chip (organ chip) microfluidic culture models of the human intestine that are lined by epithelial cells and interfaced with other tissues (such as stroma or endothelium), which can experience both fluid flow and peristalsis-like motions. Organ chips offer powerful ways to model intestinal physiology and disease states for various human populations and individual patients, and can be used to gain new insight into underlying molecular and biophysical mechanisms of disease. They can also be used as preclinical tools to discover new drugs and then validate their therapeutic efficacy and safety in the same human-relevant model. Studying human physiology and pathophysiology in preclinical animal models has drawbacks. Developments in organ-on-a-chip (organ chip) technology have opened up new ways to model human intestinal physiology. This Review discusses intestinal organ chips as disease models and preclinical tools and their potential for personalized medicine.
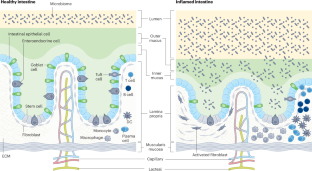
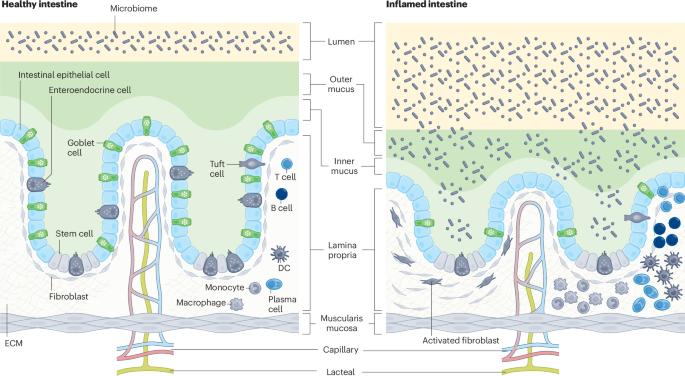
用于疾病建模和个性化医疗的肠道器官芯片。
肠道结构、力学和生理学的改变是急性和慢性肠道疾病的基础,其中许多疾病受到微生物组、蠕动、基质或免疫反应失调的影响。临床前动物模型很难研究人类肠道生理学或病理生理学,因为它们的微生物组和免疫系统与人类不同。虽然类器官培养技术的进步部分克服了这一难题,但肠类器官仍然缺乏研究肠道健康和疾病的核心功能(如消化或液体流动)所必需的关键特征,以及活体微生物组、蠕动和免疫细胞的长期影响所带来的贡献。在这里,我们将回顾器官芯片(organ chip)微流体培养人体肠道模型的发展,这些模型由上皮细胞衬里,并与其他组织(如基质或内皮细胞)相连接,可以经历液体流动和类似蠕动的运动。器官芯片为模拟不同人群和个体患者的肠道生理和疾病状态提供了强有力的方法,可用于深入了解疾病的潜在分子和生物物理机制。它们还可用作发现新药的临床前工具,然后在相同的人体相关模型中验证其疗效和安全性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
52.30
自引率
0.60%
发文量
147
审稿时长
6-12 weeks
期刊介绍:
Nature Reviews Gastroenterology & Hepatology aims to serve as the leading resource for Reviews and commentaries within the scientific and medical communities it caters to. The journal strives to maintain authority, accessibility, and clarity in its published articles, which are complemented by easily understandable figures, tables, and other display items. Dedicated to providing exceptional service to authors, referees, and readers, the editorial team works diligently to maximize the usefulness and impact of each publication.
The journal encompasses a wide range of content types, including Research Highlights, News & Views, Comments, Reviews, Perspectives, and Consensus Statements, all pertinent to gastroenterologists and hepatologists. With its broad scope, Nature Reviews Gastroenterology & Hepatology ensures that its articles reach a diverse audience, aiming for the widest possible dissemination of valuable information.
Nature Reviews Gastroenterology & Hepatology is part of the Nature Reviews portfolio of journals.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


