TFAP2A Activates S100A2 to Mediate Glutamine Metabolism and Promote Lung Adenocarcinoma Metastasis
Abstract
Background
Lung adenocarcinoma (LUAD) is a fatal disease with metabolic abnormalities. The dysregulation of S100 calcium-binding protein A2 (S100A2), a member of the S100 protein family, is connected to the development of various cancers. The impact of S100A2 on the LUAD occurrence and metastasis, however, has not yet been reported. The functional mechanism of S100A2 on LUAD cell metastasis was examined in this article.
Methods
The expression of TFAP2A and S100A2 in LUAD tissues and cells was analyzed by bioinformatics and qRT-PCR, respectively. The enrichment pathway analysis was performed on S100A2. Bioinformatics analysis determined the binding relationship between TFAP2A and S100A2, and their interaction was validated through dual-luciferase and chromatin immunoprecipitation experiments. Cell viability was determined using cell counting kit-8 (CCK-8). A transwell assay was performed to analyze the invasion and migration of cells. Immunofluorescence was conducted to obtain vimentin and E-cadherin expression, and a western blot was used to detect the expression of MMP-2, MMP-9, GLS, and GLUD1. The kits measured the NADPH/NADP ratio, glutathione (GSH)/glutathione disulfide (GSSG) levels, and the contents of glutamine, α-KG, and glutamate.
Results
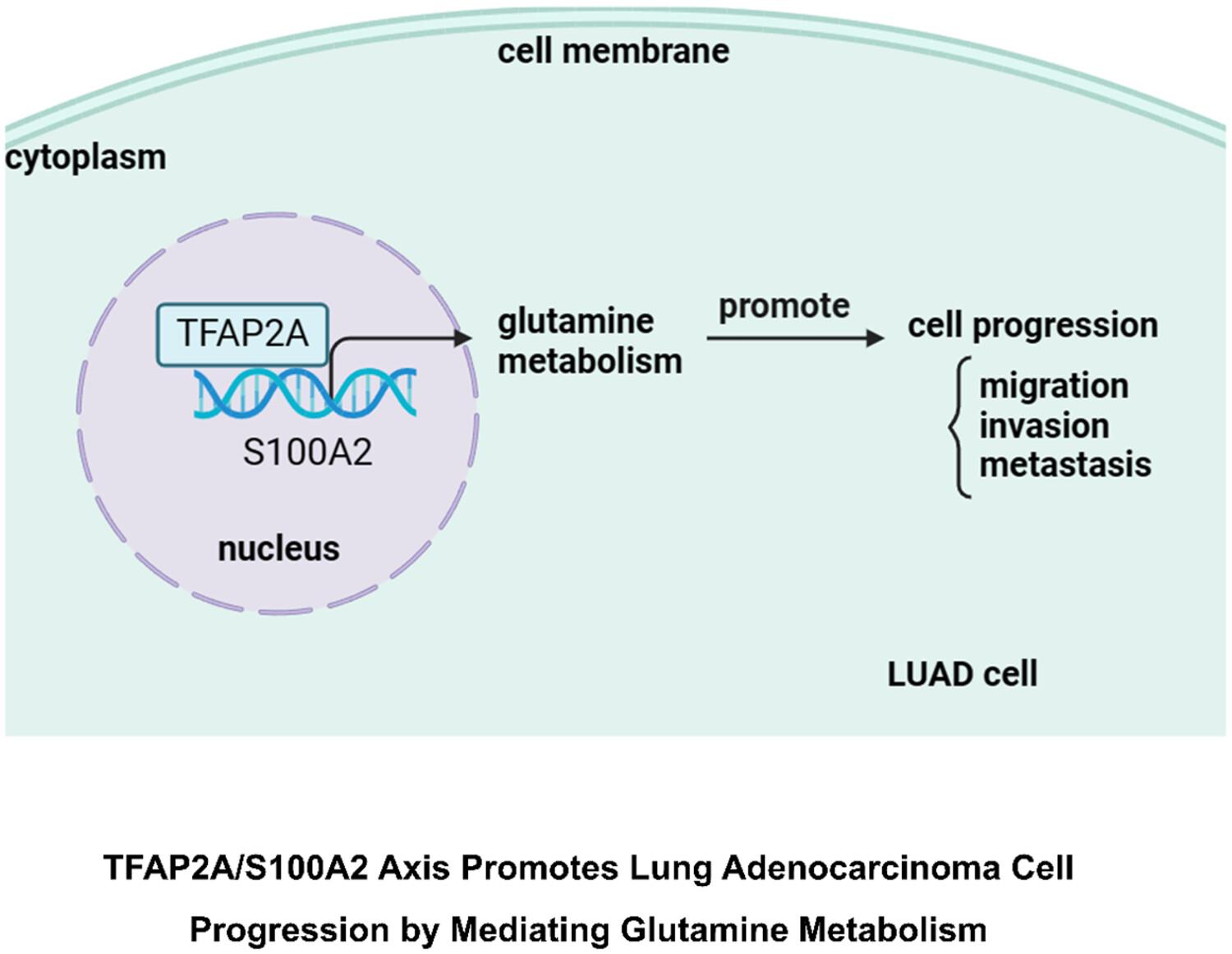
S100A2 was upregulated in LUAD tissues and cells, and S100A2 mediated glutamine metabolism to induce LUAD metastasis. Additionally, the transcriptional regulator TFAP2A was discovered upstream of S100A2, and TFAP2A expression was upregulated in LUAD, which indicated that TFAP2A promoted the S100A2 expression. The rescue experiment found that upregulation of S100A2 could reverse the inhibitory effects of silencing TFAP2A on glutamine metabolism and cell metastasis.
Conclusion
In conclusion, by regulating glutamine metabolism, the TFAP2A/S100A2 axis facilitated LUAD metastasis. This suggested that targeting S100A2 could be beneficial for LUAD treatment.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


