Standardizing designed and emergent quantitative features in microphysiological systems
IF 26.8
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
Microphysiological systems (MPSs) are cellular models that replicate aspects of organ and tissue functions in vitro. In contrast with conventional cell cultures, MPSs often provide physiological mechanical cues to cells, include fluid flow and can be interlinked (hence, they are often referred to as microfluidic tissue chips or organs-on-chips). Here, by means of examples of MPSs of the vascular system, intestine, brain and heart, we advocate for the development of standards that allow for comparisons of quantitative physiological features in MPSs and humans. Such standards should ensure that the in vivo relevance and predictive value of MPSs can be properly assessed as fit-for-purpose in specific applications, such as the assessment of drug toxicity, the identification of therapeutics or the understanding of human physiology or disease. Specifically, we distinguish designed features, which can be controlled via the design of the MPS, from emergent features, which describe cellular function, and propose methods for improving MPSs with readouts and sensors for the quantitative monitoring of complex physiology towards enabling wider end-user adoption and regulatory acceptance. This Perspective discusses the need for standards that allow for comparisons of quantitative physiological features in microphysiological systems and humans.
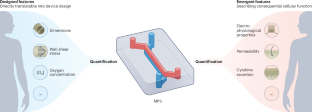
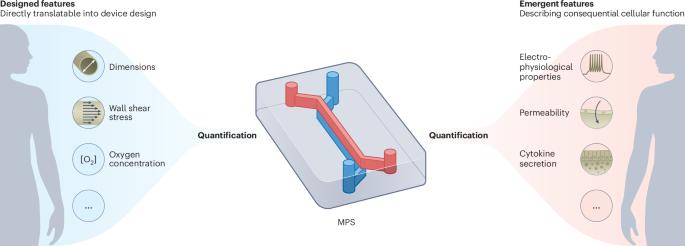
将微观生理系统中设计和出现的定量特征标准化。
微生理系统(MPS)是在体外复制器官和组织功能的细胞模型。与传统的细胞培养不同,微物理系统通常为细胞提供生理机械线索,包括液体流动,并且可以相互连接(因此,它们通常被称为微流体组织芯片或芯片上的器官)。在此,我们以血管系统、肠道、大脑和心脏的微流体组织芯片为例,主张制定标准,以便对微流体组织芯片和人体的定量生理特征进行比较。此类标准应确保 MPS 的体内相关性和预测价值能够在特定应用中得到适当评估,如评估药物毒性、确定治疗方法或了解人体生理或疾病。具体来说,我们将可通过 MPS 设计进行控制的设计特征与描述细胞功能的突发特征区分开来,并提出了改进带有读数器和传感器的 MPS 的方法,以便对复杂的生理学进行定量监测,从而使最终用户更广泛地采用 MPS 并获得监管部门的认可。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Biomedical Engineering
Medicine-Medicine (miscellaneous)
CiteScore
45.30
自引率
1.10%
发文量
138
期刊介绍:
Nature Biomedical Engineering is an online-only monthly journal that was launched in January 2017. It aims to publish original research, reviews, and commentary focusing on applied biomedicine and health technology. The journal targets a diverse audience, including life scientists who are involved in developing experimental or computational systems and methods to enhance our understanding of human physiology. It also covers biomedical researchers and engineers who are engaged in designing or optimizing therapies, assays, devices, or procedures for diagnosing or treating diseases. Additionally, clinicians, who make use of research outputs to evaluate patient health or administer therapy in various clinical settings and healthcare contexts, are also part of the target audience.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


