Plasmon-driven chemical transformation of a secondary amide probed by surface enhanced Raman scattering
IF 5.9
2区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Plasmon-driven chemical conversion is gaining burgeoning interest in the field of heterogeneous catalysis. Herein, we study the reactivity of N-methyl-4-sulfanylbenzamide (NMSB) at nanocavities of gold and silver nanoparticle aggregates under plasmonic excitation to gain understanding of the respective reaction mechanism. NMSB is a secondary amide, which is a frequent binding motive found in peptides and a common coupling product of organic molecules and biomolecules. Surface-enhanced Raman scattering (SERS) is used as a two-in-one in-situ spectroscopic tool to initiate the molecular transformation process and simultaneously monitor and analyze the reaction products. Supported by dissociative electron attachment (DEA) studies with the gas phase molecule, a hot electron-mediated conversion of NMSB to p-mercaptobenzamide and p-mercaptobenzonitrile is proposed at the plasmonic nanocavities. The reaction rate showed negligible dependence on the external temperature, ruling out the dominant role of heat in the chemical transformation at the plasmonic interface. This is reflected in the absence of a superlinear relationship between the reaction rate constant and the laser power density, and DEA and SERS studies indicate a hot-electron mediated pathway. We conclude that the overall reaction rate is limited by the availability of energetic hot electrons to the NMSB molecule. Photocatalysis driven by surface plasmons is a promising approach for light-driven chemical conversions of molecules under mild conditions. Here, the authors study the reactivity of N-methyl-4-sulfanylbenzamide (NMSB) at the nanocavities of gold and silver nanoparticle aggregates under plasmonic excitation and propose a hot electron-mediated conversion of NMSB to p-mercaptobenzamide and p-mercaptobenzonitrile.
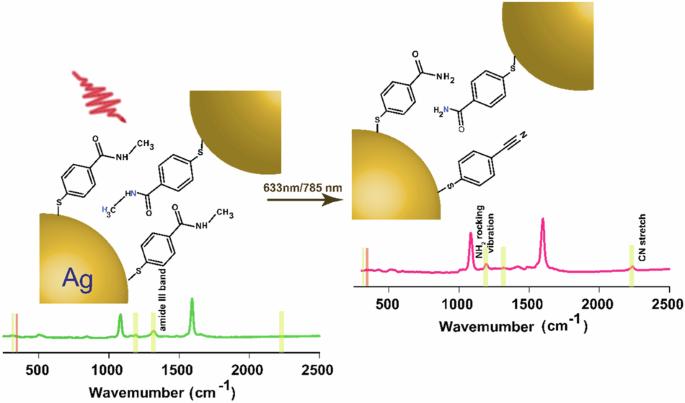
通过表面增强拉曼散射探测仲酰胺的等离子体驱动化学变化。
等离子体驱动的化学转化在异相催化领域越来越受到关注。在此,我们研究了 N-甲基-4-硫代苯甲酰胺(NMSB)在质子激发下在金纳米粒子纳米腔和银纳米粒子聚集体上的反应性,以了解各自的反应机理。NMSB 是一种仲酰胺,是肽中常见的结合动机,也是有机分子与生物大分子的常见偶联产物。表面增强拉曼散射(SERS)是一种二合一的原位光谱工具,可用于启动分子转化过程,并同时监测和分析反应产物。在气相分子离解电子附着(DEA)研究的支持下,提出了在质子纳米腔内由热电子介导的 NMSB 向对巯基苯甲酰胺和对巯基苯腈的转化过程。反应速率与外部温度的关系微乎其微,这就排除了热量在质子界面化学转化中的主导作用。这反映在反应速率常数与激光功率密度之间没有超线性关系,而 DEA 和 SERS 研究表明了热电子介导的途径。我们的结论是,整个反应速率受 NMSB 分子能否获得高能热电子的限制。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Communications Chemistry
Chemistry-General Chemistry
CiteScore
7.70
自引率
1.70%
发文量
146
审稿时长
13 weeks
期刊介绍:
Communications Chemistry is an open access journal from Nature Research publishing high-quality research, reviews and commentary in all areas of the chemical sciences. Research papers published by the journal represent significant advances bringing new chemical insight to a specialized area of research. We also aim to provide a community forum for issues of importance to all chemists, regardless of sub-discipline.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


