Indoline hemiaminals: a platform for accessing anthranilic acid derivatives through oxidative deformylation†
IF 2.7
3区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
2-Aminobenzoyl chlorides possess both a nucleophilic nitrogen atom and an electrophilic carbonyl group, and thus selective acylation of nucleophiles is challenging; self-dimerization and sluggish reactions occur. Herein, we introduce a new synthetic protocol using 2-aminobenzoyl surrogates, allowing concise entry to decorated 2-aminobenzoyl derivatives in the absence of transition metals, acid chlorides, and specific reagents.
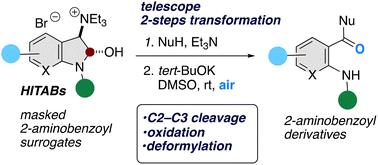
吲哚啉半二胺:通过氧化变形获得花苯胺酸衍生物的平台。
2- 氨基苯甲酰氯同时具有亲核的氮原子和亲电的羰基,因此亲核物的选择性酰化具有挑战性,会出现自嵌合和反应迟缓的现象。在此,我们介绍了一种使用 2-氨基苯甲酰基替代物的新合成方案,在没有过渡金属、酸性氯化物和特定试剂的情况下,可以简便地进入装饰的 2-氨基苯甲酰基衍生物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic & Biomolecular Chemistry
化学-有机化学
CiteScore
5.50
自引率
9.40%
发文量
1056
审稿时长
1.3 months
期刊介绍:
Organic & Biomolecular Chemistry is an international journal using integrated research in chemistry-organic chemistry. Founded in 2003 by the Royal Society of Chemistry, the journal is published in Semimonthly issues and has been indexed by SCIE, a leading international database. The journal focuses on the key research and cutting-edge progress in the field of chemistry-organic chemistry, publishes and reports the research results in this field in a timely manner, and is committed to becoming a window and platform for rapid academic exchanges among peers in this field. The journal's impact factor in 2023 is 2.9, and its CiteScore is 5.5.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


