High-fat feeding drives the intestinal production and assembly of C16:0 ceramides in chylomicrons
IF 11.7
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
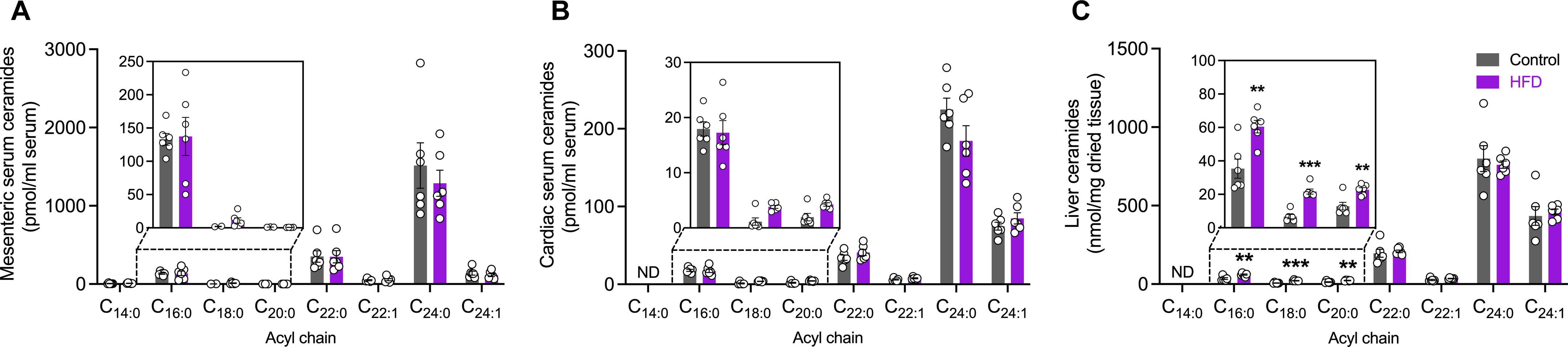
Consumption of a diet rich in saturated fat increases lipid absorption from the intestine, assembly into chylomicrons, and delivery to metabolic tissues via the lymphatic and circulatory systems. Accumulation of ceramide lipids, composed of sphingosine and a fatty acid, in metabolic tissues contributes to the pathogenesis of cardiovascular diseases, type 2 diabetes mellitus and cancer. Using a mesenteric lymph duct cannulated rat model, we showed that ceramides are generated by the intestine and assembled into chylomicrons, which are transported via the mesenteric lymphatic system. A lipidomic screen of intestinal-derived chylomicrons identified a diverse range of fatty acid, sphingolipid, and glycerolipid species that have not been previously detected in chylomicrons, including the metabolically deleterious C16:0 ceramide that increased in response to high-fat feeding in rats and human high-lipid meal replacement enteral feeding. In conclusion, high-fat feeding increases the export of intestinal-derived C16:0 ceramide in chylomicrons, identifying a potentially unknown mechanism through which ceramides are transported systemically to contribute to metabolic dysfunction.
高脂肪摄入会促使肠道产生C16:0神经酰胺并在乳糜微粒中组装。
摄入富含饱和脂肪的饮食会增加肠道对脂质的吸收,使其形成乳糜微粒,并通过淋巴和循环系统输送到代谢组织。由鞘氨醇和一种脂肪酸组成的神经酰胺脂质在代谢组织中的积累是心血管疾病、2 型糖尿病和癌症的发病机理之一。利用肠系膜淋巴管插管大鼠模型,我们发现神经酰胺由肠道生成并组装成乳糜微粒,然后通过肠系膜淋巴系统运输。通过对肠源性乳糜微粒进行脂质组学筛选,发现了乳糜微粒中以前未曾检测到的多种脂肪酸、鞘脂和甘油脂类,包括对代谢有害的 C16:0 神经酰胺,这种神经酰胺在大鼠高脂喂养和人类高脂替代餐肠内喂养时会增加。总之,高脂喂养增加了乳糜微粒中肠源性 C16:0 神经酰胺的输出,确定了神经酰胺通过系统运输导致代谢功能障碍的潜在未知机制。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Science Advances
综合性期刊-综合性期刊
CiteScore
21.40
自引率
1.50%
发文量
1937
审稿时长
29 weeks
期刊介绍:
Science Advances, an open-access journal by AAAS, publishes impactful research in diverse scientific areas. It aims for fair, fast, and expert peer review, providing freely accessible research to readers. Led by distinguished scientists, the journal supports AAAS's mission by extending Science magazine's capacity to identify and promote significant advances. Evolving digital publishing technologies play a crucial role in advancing AAAS's global mission for science communication and benefitting humankind.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


