Acid-degradable lipid nanoparticles enhance the delivery of mRNA
IF 38.1
1区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
Abstract
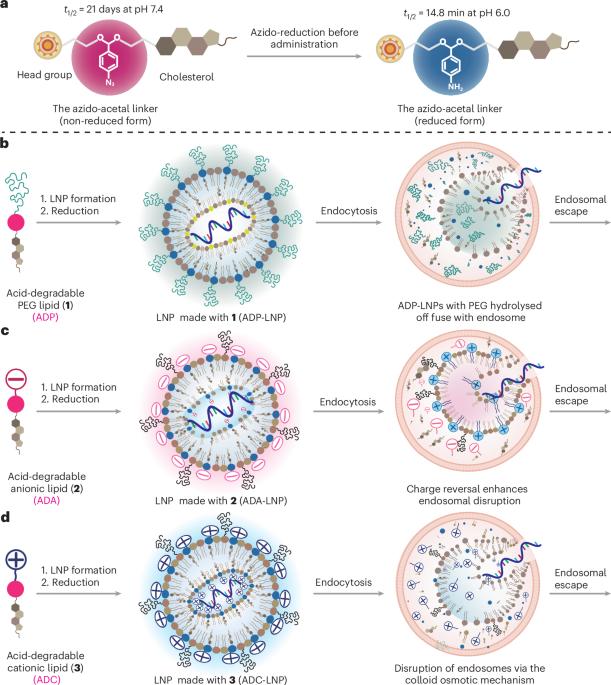
Lipid nanoparticle (LNP)–mRNA complexes are transforming medicine. However, the medical applications of LNPs are limited by their low endosomal disruption rates, high toxicity and long tissue persistence times. LNPs that rapidly hydrolyse in endosomes (RD-LNPs) could solve the problems limiting LNP-based therapeutics and dramatically expand their applications but have been challenging to synthesize. Here we present an acid-degradable linker termed ‘azido-acetal’ that hydrolyses in endosomes within minutes and enables the production of RD-LNPs. Acid-degradable lipids composed of polyethylene glycol lipids, anionic lipids and cationic lipids were synthesized with the azido-acetal linker and used to generate RD-LNPs, which significantly improved the performance of LNP–mRNA complexes in vitro and in vivo. Collectively, RD-LNPs delivered mRNA more efficiently to the liver, lung, spleen and brains of mice and to haematopoietic stem and progenitor cells in vitro than conventional LNPs. These experiments demonstrate that engineering LNP hydrolysis rates in vivo has great potential for expanding the medical applications of LNPs. A new acid-degradable linker termed ‘azido-acetal’ has been developed that rapidly hydrolyses at pH 6.0 but is stable at pH 7.4. Lipid nanoparticles made with this linker delivered mRNA in vivo and in vitro better than traditional lipid nanoparticles.
酸性可降解脂质纳米颗粒可增强 mRNA 的传递
脂质纳米粒子(LNP)-mRNA 复合物正在改变医学。然而,LNPs 的医疗应用受到其内体破坏率低、毒性大和组织持久时间长的限制。能在内质体中快速水解的 LNPs(RD-LNPs)可以解决限制基于 LNP 的疗法的问题,并极大地扩展其应用范围,但合成 LNPs 却具有挑战性。在这里,我们介绍了一种被称为 "叠氮缩醛 "的可酸降解连接体,它能在数分钟内在内体中水解,从而生产出 RD-LNPs。由聚乙二醇脂质、阴离子脂质和阳离子脂质组成的酸可降解脂质与叠氮-缩醛连接体合成,并用于生成 RD-LNPs,从而显著提高了 LNP-mRNA 复合物在体外和体内的性能。总之,与传统的 LNPs 相比,RD-LNPs 能更有效地将 mRNA 运送到小鼠的肝、肺、脾和大脑,并在体外运送到造血干细胞和祖细胞。这些实验证明,在体内设计 LNP 的水解率对于扩大 LNP 的医学应用具有巨大潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature nanotechnology
工程技术-材料科学:综合
CiteScore
59.70
自引率
0.80%
发文量
196
审稿时长
4-8 weeks
期刊介绍:
Nature Nanotechnology is a prestigious journal that publishes high-quality papers in various areas of nanoscience and nanotechnology. The journal focuses on the design, characterization, and production of structures, devices, and systems that manipulate and control materials at atomic, molecular, and macromolecular scales. It encompasses both bottom-up and top-down approaches, as well as their combinations.
Furthermore, Nature Nanotechnology fosters the exchange of ideas among researchers from diverse disciplines such as chemistry, physics, material science, biomedical research, engineering, and more. It promotes collaboration at the forefront of this multidisciplinary field. The journal covers a wide range of topics, from fundamental research in physics, chemistry, and biology, including computational work and simulations, to the development of innovative devices and technologies for various industrial sectors such as information technology, medicine, manufacturing, high-performance materials, energy, and environmental technologies. It includes coverage of organic, inorganic, and hybrid materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


