Mapping neuroimmune interactions in the gut
IF 45.9
1区 医学
Q1 GASTROENTEROLOGY & HEPATOLOGY
引用次数: 0
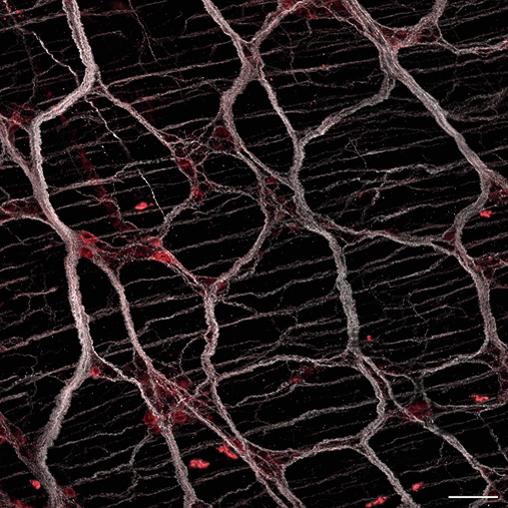
绘制肠道神经免疫相互作用图
新研究绘制了小鼠肠道中的神经免疫相互作用图,揭示了表达TRPV1的痛觉感受器神经元(参与内脏疼痛)控制和抑制肠道中的调节性T(Treg)细胞,增加了小鼠模型对结肠炎的易感性。在他们的筛选中,小鼠的八个不同神经元亚群被激活并绘制了图谱。在他们的筛选过程中,激活了小鼠体内八个不同的神经元亚群并绘制了图谱,在激活后观察到了不同的免疫干扰:硝酸神经元(NOS1表达)调节回肠中的T辅助17样细胞;胆碱能神经元(表达胆碱乙酰转移酶)调节回肠中性粒细胞;痛觉神经元(TRPV1表达)调节一系列细胞类型,包括2型先天性淋巴细胞、活化的CD8+ T细胞、巨噬细胞和RORγ Treg细胞。对痛觉神经元进行更详细的研究发现,背根神经节中的TRPV1+神经元通过降钙素基因相关肽抑制结肠中的RORγ+ Treg细胞,而降钙素基因相关肽是通过受体RAMP1-CALCRL介导的。重要的是,TRPV1+神经元的激活和随后对Treg细胞的下调导致小鼠对肠道炎症的易感性增加(通过柠檬酸杆菌感染和服用葡聚糖硫酸钠诱导)。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
52.30
自引率
0.60%
发文量
147
审稿时长
6-12 weeks
期刊介绍:
Nature Reviews Gastroenterology & Hepatology aims to serve as the leading resource for Reviews and commentaries within the scientific and medical communities it caters to. The journal strives to maintain authority, accessibility, and clarity in its published articles, which are complemented by easily understandable figures, tables, and other display items. Dedicated to providing exceptional service to authors, referees, and readers, the editorial team works diligently to maximize the usefulness and impact of each publication.
The journal encompasses a wide range of content types, including Research Highlights, News & Views, Comments, Reviews, Perspectives, and Consensus Statements, all pertinent to gastroenterologists and hepatologists. With its broad scope, Nature Reviews Gastroenterology & Hepatology ensures that its articles reach a diverse audience, aiming for the widest possible dissemination of valuable information.
Nature Reviews Gastroenterology & Hepatology is part of the Nature Reviews portfolio of journals.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


