The Rubicon–WIPI axis regulates exosome biogenesis during ageing
IF 17.3
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
Abstract
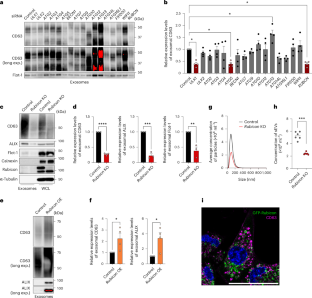
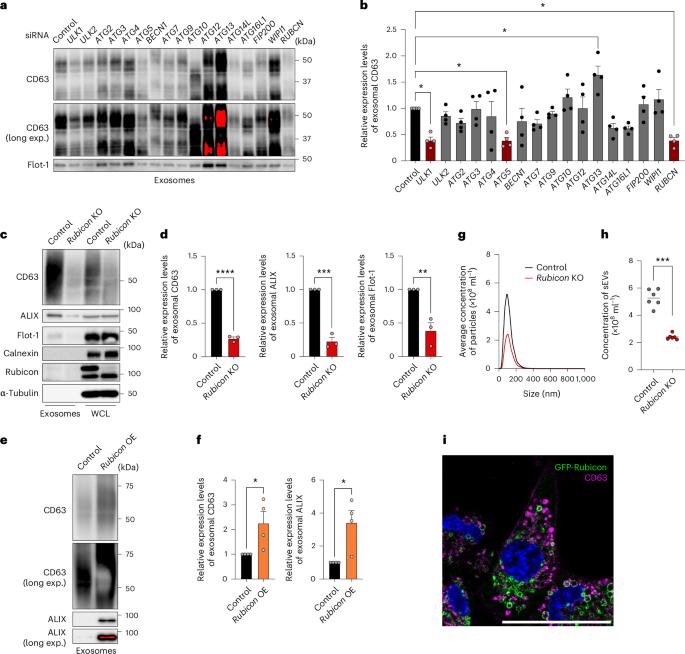
Cells release intraluminal vesicles in multivesicular bodies as exosomes to communicate with other cells. Although recent studies suggest an intimate link between exosome biogenesis and autophagy, the detailed mechanism is not fully understood. Here we employed comprehensive RNA interference screening for autophagy-related factors and discovered that Rubicon, a negative regulator of autophagy, is essential for exosome release. Rubicon recruits WIPI2d to endosomes to promote exosome biogenesis. Interactome analysis of WIPI2d identified the ESCRT components that are required for intraluminal vesicle formation. Notably, we found that Rubicon is required for an age-dependent increase of exosome release in mice. In addition, small RNA sequencing of serum exosomes revealed that Rubicon determines the fate of exosomal microRNAs associated with cellular senescence and longevity pathways. Taken together, our current results suggest that the Rubicon–WIPI axis functions as a key regulator of exosome biogenesis and is responsible for age-dependent changes in exosome quantity and quality. Yanagawa et al. show that the autophagy-related protein Rubicon recruits WIPI2d to endosomes to promote exosome biogenesis. Rubicon promotes both an increase in exosome release during ageing and the pro-senescent effects of these exosomes.
Rubicon-WIPI轴调节衰老过程中的外泌体生物生成
细胞释放多囊体中的腔内囊泡作为外泌体,与其他细胞进行交流。尽管最近的研究表明外泌体的生物生成与自噬之间存在密切联系,但其详细机制尚未完全明了。在这里,我们对自噬相关因子进行了全面的RNA干扰筛选,发现自噬的负调控因子Rubicon对外泌体的释放至关重要。Rubicon 将 WIPI2d 募集到内体以促进外泌体的生物生成。WIPI2d的相互作用组分析确定了腔内囊泡形成所需的ESCRT成分。值得注意的是,我们发现 Rubicon 是小鼠外泌体释放随年龄增长而增加的必要条件。此外,血清外泌体的小 RNA 测序显示,Rubicon 决定着与细胞衰老和长寿途径相关的外泌体 microRNA 的命运。综上所述,我们目前的研究结果表明,Rubicon-WIPI 轴是外泌体生物生成的关键调节因子,是外泌体数量和质量随年龄变化的原因。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
Nature Cell Biology
生物-细胞生物学
CiteScore
28.40
自引率
0.90%
发文量
219
审稿时长
3 months
期刊介绍:
Nature Cell Biology, a prestigious journal, upholds a commitment to publishing papers of the highest quality across all areas of cell biology, with a particular focus on elucidating mechanisms underlying fundamental cell biological processes. The journal's broad scope encompasses various areas of interest, including but not limited to:
-Autophagy
-Cancer biology
-Cell adhesion and migration
-Cell cycle and growth
-Cell death
-Chromatin and epigenetics
-Cytoskeletal dynamics
-Developmental biology
-DNA replication and repair
-Mechanisms of human disease
-Mechanobiology
-Membrane traffic and dynamics
-Metabolism
-Nuclear organization and dynamics
-Organelle biology
-Proteolysis and quality control
-RNA biology
-Signal transduction
-Stem cell biology
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


