NIR-II triggered Cu(I) phosphide for chemodynamic and photothermal periodontitis treatment: Efficient reduction of bacterial co-aggregation
IF 9.4
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
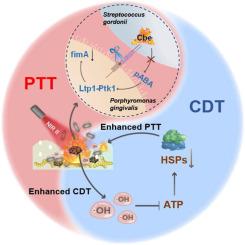
The synergy between chemodynamic therapy (CDT) and photothermal therapy (PTT) offers a promising antimicrobial strategy for periodontitis, yet faces challenges like complex material structure and limited NIR-I light penetration. Additionally, low endogenous H2O2 levels in biofilm and a focus on bacterial eradication over colonization prevention limit current treatments. To address these issues, we newly introduce a single-material system (Cu3P@PAH@Lox) that integrates dual functionalities to synergistically enhance antimicrobial effects and significantly reduce pathogen co-aggregation. This system utilizes PTT to increase local temperature, boosting •OH production in CDT while downregulating heat shock proteins to enhance PTT efficacy, forming a self-reinforcing feedback loop. Lactate oxidase (Lox) is employed to convert lactate—a metabolite in periodontal biofilm—into H2O2, further amplifying CDT's potential. In vitro Cu3P@PAH@Lox demonstrates a remarkable synergistic effect against dual-species biofilms by more than 2-log reduction of colony-forming unit. Moreover, Cu3P@PAH@Lox exhibits outstanding synergistic antibacterial performances to alleviate inflammation and destruction of tissue in vivo periodontitis model. Furthermore, the mechanism of pathogen co-aggregation disruption by PTT is verified via the Cbe-Ltp1-Ptk1-fimA signaling pathway. This single-material multimodal system we have herein demonstrated for the first time marks a significant advancement in periodontitis treatment, eradicating microbes and preventing bacterial colonization, offering a path to comprehensive periodontal care.
Statement of significance
The synergy between chemodynamic therapy (CDT) and photothermal therapy (PTT) has been considered a promising therapy for periodontitis. Yet, facing challenges, the complex material structure, limited NIR-I light penetration, low endogenous H2O2 level in biofilm, and a focus on bacterial eradication over colonization prevention are still insufficient. This study pioneers a unique, single-material system (Cu3P@PAH@Lox) that synergistically enhances antimicrobial effects and substantially curtails pathogen co-aggregation, advancing periodontitis therapy. By exploiting PTT to elevate local temperatures, thereby increasing hydroxyl radical production in CDT and concurrently suppressing heat shock proteins, the system establishes a potent, self-enhancing loop. Furthermore, lactate oxidase is innovatively utilized to convert lactate from periodontal biofilm into hydrogen peroxide, augmenting the efficacy of CDT. The introduction of Cu3P@PAH@Lox is poised to revolutionize periodontitis treatment, eliminating microbes and impeding bacterial colonization, thereby charting a course for comprehensive periodontal management.
近红外-II触发磷化铜(I)用于化学动力和光热治疗牙周炎:有效减少细菌共聚集。
化学动力疗法(CDT)和光热疗法(PTT)的协同作用为牙周炎提供了一种前景广阔的抗菌策略,但也面临着材料结构复杂、近红外光穿透力有限等挑战。此外,生物膜中的内源性 H2O2 水平较低,以及只注重根除细菌而忽视预防定植,也限制了目前的治疗方法。为了解决这些问题,我们新近推出了一种单一材料系统(Cu3P@PAH@Lox),该系统集成了双重功能,可协同增强抗菌效果并显著减少病原体的共聚集。该系统利用 PTT 提高局部温度,促进 CDT 中 -OH 的产生,同时下调热休克蛋白以增强 PTT 的功效,从而形成一个自我强化的反馈回路。乳酸氧化酶(Lox)被用来将乳酸(牙周生物膜中的代谢物)转化为 H2O2,进一步增强 CDT 的潜力。体外 Cu3P@PAH@Lox 对双物种生物膜具有显著的协同作用,菌落形成单位减少了 2 个以上的菌落。此外,在活体牙周炎模型中,Cu3P@PAH@Lox 在减轻炎症和组织破坏方面也表现出卓越的协同抗菌性能。此外,还通过 Cbe-Ltp1-Ptk1-fimA 信号通路验证了 PTT 破坏病原体共聚集的机制。我们在此首次展示的这种单一材料多模式系统标志着牙周炎治疗领域的重大进展,它能根除微生物并防止细菌定植,为牙周综合治疗提供了一条途径。意义说明:化学动力疗法(CDT)和光热疗法(PTT)之间的协同作用一直被认为是治疗牙周炎的一种前景广阔的疗法。然而,由于材料结构复杂、近红外-I 光穿透力有限、生物膜中内源性 H2O2 水平较低、重细菌根除而轻定植预防等原因,该疗法仍面临诸多挑战。本研究开创了一种独特的单一材料系统(Cu3P@PAH@Lox),它能协同增强抗菌效果,并大大减少病原体的共同聚集,从而推进牙周炎的治疗。通过利用 PTT 提高局部温度,从而增加 CDT 中羟基自由基的产生,同时抑制热休克蛋白,该系统建立了一个有效的自我增强循环。此外,乳酸氧化酶被创新性地用于将牙周生物膜中的乳酸转化为过氧化氢,从而增强 CDT 的功效。Cu3P@PAH@Lox 的问世有望彻底改变牙周炎的治疗方法,消除微生物并阻止细菌定植,从而为牙周病的综合治理指明方向。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Acta Biomaterialia
工程技术-材料科学:生物材料
CiteScore
16.80
自引率
3.10%
发文量
776
审稿时长
30 days
期刊介绍:
Acta Biomaterialia is a monthly peer-reviewed scientific journal published by Elsevier. The journal was established in January 2005. The editor-in-chief is W.R. Wagner (University of Pittsburgh). The journal covers research in biomaterials science, including the interrelationship of biomaterial structure and function from macroscale to nanoscale. Topical coverage includes biomedical and biocompatible materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


