Predicting 1, 2 and 3 year emergent referable diabetic retinopathy and maculopathy using deep learning
IF 5.4
Q1 MEDICINE, RESEARCH & EXPERIMENTAL
引用次数: 0
Abstract
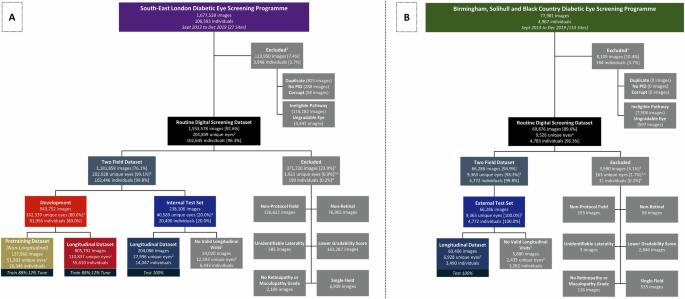
Predicting diabetic retinopathy (DR) progression could enable individualised screening with prompt referral for high-risk individuals for sight-saving treatment, whilst reducing screening burden for low-risk individuals. We developed and validated deep learning systems (DLS) that predict 1, 2 and 3 year emergent referable DR and maculopathy using risk factor characteristics (tabular DLS), colour fundal photographs (image DLS) or both (multimodal DLS). From 162,339 development-set eyes from south-east London (UK) diabetic eye screening programme (DESP), 110,837 had eligible longitudinal data, with the remaining 51,502 used for pretraining. Internal and external (Birmingham DESP, UK) test datasets included 27,996, and 6928 eyes respectively. Internal multimodal DLS emergent referable DR, maculopathy or either area-under-the receiver operating characteristic (AUROC) were 0.95 (95% CI: 0.92–0.98), 0.84 (0.82–0.86), 0.85 (0.83–0.87) for 1 year, 0.92 (0.87–0.96), 0.84 (0.82–0.87), 0.85 (0.82–0.87) for 2 years, and 0.85 (0.80–0.90), 0.79 (0.76–0.82), 0.79 (0.76–0.82) for 3 years. External multimodal DLS emergent referable DR, maculopathy or either AUROC were 0.93 (0.88–0.97), 0.85 (0.80–0.89), 0.85 (0.76–0.85) for 1 year, 0.93 (0.89–0.97), 0.79 (0.74–0.84), 0.80 (0.76–0.85) for 2 years, and 0.91 (0.84–0.98), 0.79 (0.74–0.83), 0.79 (0.74–0.84) for 3 years. Multimodal and image DLS performance is significantly better than tabular DLS at all intervals. DLS accurately predict 1, 2 and 3 year emergent referable DR and referable maculopathy using colour fundal photographs, with additional risk factor characteristics conferring improvements in prognostic performance. Proposed DLS are a step towards individualised risk-based screening, whereby AI-assistance allows high-risk individuals to be closely monitored while reducing screening burden for low-risk individuals. Diabetic retinopathy (DR) is a disease where the light-sensing layer at the back of the eye (retina) becomes damaged by raised blood sugar levels. It affects around one in three of the 463 million people with diabetes worldwide and is a leading cause of acquired vision loss in working-age adults. In this study, we developed computer-based models to predict when DR would reach a stage where vision could be threatened up to 3-years in the future. Our study shows that this system can accurately predict sight-threatening DR in patients with diabetes. This could mean fewer unnecessary visits for individuals at low-risk of DR progression, but closer monitoring and potentially earlier treatment for individuals at high-risk of DR progression, which could reduce the risk of vision loss. Nderitu et al. present deep learning systems developed to predict emergent referable diabetic retinopathy and maculopathy over 1, 2 and 3 years. Using validated tabular, image and multimodal systems they aim to individualise risk-based screening.
利用深度学习预测 1 年、2 年和 3 年的急诊可转诊糖尿病视网膜病变和黄斑病变。
背景:预测糖尿病视网膜病变(DR)的进展可以实现个体化筛查,及时转诊高危人群进行救盲治疗,同时减轻低危人群的筛查负担。我们开发并验证了深度学习系统(DLS),该系统可利用风险因素特征(表式 DLS)、彩色眼底照片(图像 DLS)或两者(多模态 DLS)预测 1、2 和 3 年内出现的可转诊 DR 和黄斑病变:从伦敦东南部(英国)糖尿病眼筛查项目(DESP)的 162,339 只发展设置眼球中,110,837 只拥有合格的纵向数据,其余 51,502 只用于预培训。内部和外部(英国伯明翰 DESP)测试数据集分别包括 27996 只眼睛和 6928 只眼睛:内部多模态 DLS 的可转诊 DR、黄斑病变或区域接收器操作特征 (AUROC) 分别为 0.95(95% CI:0.92-0.98)、0.84(0.82-0.1年为0.95(95% CI:0.92-0.98)、0.84(0.82-0.86)、0.85(0.83-0.87),2年为0.92(0.87-0.96)、0.84(0.82-0.87)、0.85(0.82-0.87),3年为0.85(0.80-0.90)、0.79(0.76-0.82)、0.79(0.76-0.82)。外部多模态 DLS 急诊可转诊的 DR、黄斑病变或任一 AUROC 1 年分别为 0.93(0.88-0.97)、0.85(0.80-0.89)、0.85(0.76-0.85),3 年分别为 0.85(0.80-0.90)、0.79(0.76-0.82)、0.79(0.76-0.82)。93(0.89-0.97)、0.79(0.74-0.84)、2 年为 0.80(0.76-0.85),3 年为 0.91(0.84-0.98)、0.79(0.74-0.83)、0.79(0.74-0.84):结论:多模态和图像 DLS 在所有时间间隔内的表现都明显优于表格 DLS。使用彩色眼底照片,DLS 可以准确预测 1 年、2 年和 3 年的急诊可转诊 DR 和可转诊黄斑病变,额外的风险因素特征可改善预后效果。拟议的 DLS 是向基于风险的个体化筛查迈出的一步,通过人工智能辅助,可以对高风险人群进行密切监测,同时减轻低风险人群的筛查负担。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


