Zinc‐Mediated Carbamoyl Amination of Alkylidenecyclopropane‐Tethered Carbamoyl Chlorides: Synthesis of Functionalized 2‐Quinolones
IF 4.4
2区 化学
Q2 CHEMISTRY, APPLIED
引用次数: 0
Abstract
The transition metal catalyzed cyclization of alkene‐tethered carbamoyl chloride has emerged as a tool to construct oxindoles bearing quaternary centers. Most of these reactions proceed via carbometalation‐initiated 5‐exo‐trig cyclization followed by nucleophilic trapping of the resulting σ alkyl‐metal species to achieve diverse functionalized oxindoles. The 6‐endo‐trig type cyclization of alkene‐tethered carbamoyl chloride has been rarely reported. Herein, a zinc‐mediated carbamoyl amination of alkylidenecyclopropane‐tethered carbamoyl chlorides with anilines for the synthesis of functionalized 2‐quinolones was developed. A range of different substituted 2‐quinolones were prepared in 65–89% yield from alkylidenecyclopropane‐tethered carbamoyl chlorides and aniline derivatives using a Zn/TMSCl system.
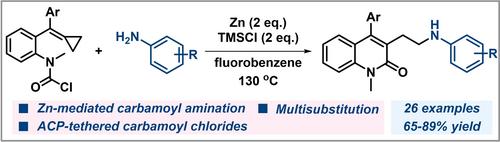
锌介导的亚烷基环丙烷系氨基甲酰基氯的氨基甲酰基胺化反应:功能化 2-喹诺酮的合成
过渡金属催化的烯系氨基甲酰氯环化反应现已成为构建含有全碳季中心的吲哚的重要工具。然而,大多数此类反应都是通过碳甲基化引发的 5-外-三位环化进行的,然后对所产生的 σ 烷基金属物种进行亲核捕获,以获得各种官能化的羰基吲哚。烯系氨基甲酰氯的 6-endo-trig 型环化反应鲜有报道。在此,我们开发了一种锌介导的氨基甲酰基氨基甲酰基胺化亚烷基环丙烷系氨基甲酰氯与苯胺的方法,用于合成官能化 2-喹啉酮。利用 Zn/TMSCl 体系从亚烷基环丙烷系氨基甲酰氯和苯胺衍生物中制备了一系列不同取代的 2-喹诺酮,收率为 65-89%。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Advanced Synthesis & Catalysis
化学-应用化学
CiteScore
9.40
自引率
7.40%
发文量
447
审稿时长
1.8 months
期刊介绍:
Advanced Synthesis & Catalysis (ASC) is the leading primary journal in organic, organometallic, and applied chemistry.
The high impact of ASC can be attributed to the unique focus of the journal, which publishes exciting new results from academic and industrial labs on efficient, practical, and environmentally friendly organic synthesis. While homogeneous, heterogeneous, organic, and enzyme catalysis are key technologies to achieve green synthesis, significant contributions to the same goal by synthesis design, reaction techniques, flow chemistry, and continuous processing, multiphase catalysis, green solvents, catalyst immobilization, and recycling, separation science, and process development are also featured in ASC. The Aims and Scope can be found in the Notice to Authors or on the first page of the table of contents in every issue.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


