Human AKR1C3 binds agonists of GPR84 and participates in an expanded polyamine pathway
IF 7.2
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Altered human aldo-keto reductase family 1 member C3 (AKR1C3) expression has been associated with poor prognosis in diverse cancers, ferroptosis resistance, and metabolic diseases. Despite its clinical significance, the endogenous biochemical roles of AKR1C3 remain incompletely defined. Using untargeted metabolomics, we identified a major transformation mediated by AKR1C3, in which a spermine oxidation product “sperminal” is reduced to “sperminol.” Sperminal causes DNA damage and activates the DNA double-strand break response, whereas sperminol induces autophagy in vitro. AKR1C3 also pulls down acyl-pyrones and pyrone-211 inhibits AKR1C3 activity. Through G protein-coupled receptor ligand screening, we determined that pyrone-211 is also a potent agonist of the semi-orphan receptor GPR84. Strikingly, mammalian fatty acid synthase produces acyl-pyrones in vitro, and this production is modulated by NADPH. Taken together, our studies support a regulatory role of AKR1C3 in an expanded polyamine pathway and a model linking fatty acid synthesis and NADPH levels to GPR84 signaling.
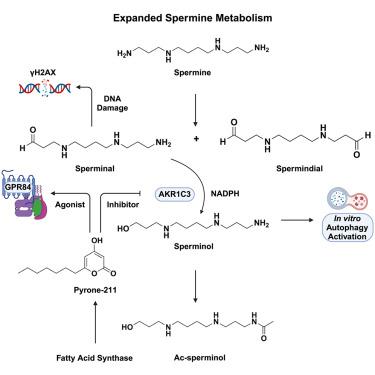

人类 AKR1C3 与 GPR84 的激动剂结合,并参与扩展的多胺途径。
人类醛酮还原酶家族 1 成员 C3(AKR1C3)表达的改变与多种癌症的不良预后、铁中毒抵抗和代谢性疾病有关。尽管AKR1C3具有重要的临床意义,但其内源生化作用仍未完全明确。利用非靶向代谢组学,我们发现了 AKR1C3 介导的一种主要转化,其中精胺氧化产物 "精胺 "被还原为 "精胺醇"。精胺会导致DNA损伤并激活DNA双链断裂反应,而精胺醇则会在体外诱导自噬。AKR1C3 还能拉低酰基吡喃酮,而吡喃酮-211 能抑制 AKR1C3 的活性。通过 G 蛋白偶联受体配体筛选,我们确定 pyrone-211 也是半orphan 受体 GPR84 的强效激动剂。令人吃惊的是,哺乳动物脂肪酸合成酶在体外产生酰基吡咯酮,而这种产生受 NADPH 的调节。综上所述,我们的研究支持 AKR1C3 在扩展的多胺通路中的调控作用,以及将脂肪酸合成和 NADPH 水平与 GPR84 信号联系起来的模型。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell Chemical Biology
Biochemistry, Genetics and Molecular Biology-Molecular Medicine
CiteScore
14.70
自引率
2.30%
发文量
143
期刊介绍:
Cell Chemical Biology, a Cell Press journal established in 1994 as Chemistry & Biology, focuses on publishing crucial advances in chemical biology research with broad appeal to our diverse community, spanning basic scientists to clinicians. Pioneering investigations at the chemistry-biology interface, the journal fosters collaboration between these disciplines. We encourage submissions providing significant conceptual advancements of broad interest across chemical, biological, clinical, and related fields. Particularly sought are articles utilizing chemical tools to perturb, visualize, and measure biological systems, offering unique insights into molecular mechanisms, disease biology, and therapeutics.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


