Direct C−C Bond Cleavage of CH3CN as a Single‐Carbon Synthon: Synthesis of Pyrrolo[1,2‐a]quinoxalines via Electrochemical Oxidation
IF 4.4
2区 化学
Q2 CHEMISTRY, APPLIED
引用次数: 0
Abstract
A direct electrochemical redox reaction involving radical cross‐coupling cyclization for the synthesis of pyrrolo[1,2‐a]quinoxaline derivatives from 1‐(2‐aminophenyl)pyrroles and CH3CN has been developed, which includes the functionalization of C(sp3)−H bonds as well as the construction of C−C and C−N bonds. Notably, the control and deuterium‐labelling experiments suggest that CH3CN in this reaction acts as both a carbon source via C−C cleavage and solvent. The reaction features metal‐ and oxidant‐free conditions, and various substituted pyrrolo[1,2‐a]quinoxaline derivatives were obtained.
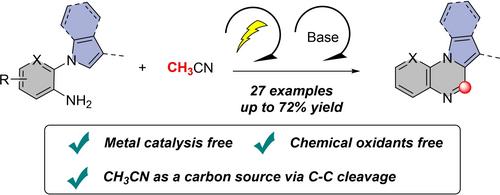
单碳合成物 CH3CN 的直接 C-C 键裂解:通过电化学氧化合成吡咯并[1,2-a]喹喔啉类化合物
研究人员开发了一种直接电化学氧化还原反应,该反应涉及自由基交叉耦合环化,用于从 1-(2-氨基苯基)吡咯和 CH3CN 合成吡咯并[1,2-a]喹喔啉衍生物,其中包括 C(sp3)-H 键的官能化以及 C-C 和 C-N 键的构建。值得注意的是,对照和氘标记实验表明,CH3CN 在该反应中通过 C-C 裂解既是碳源,又是溶剂。该反应以无金属和氧化剂条件为特征,并获得了各种取代的吡咯并[1,2-a]喹喔啉衍生物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Advanced Synthesis & Catalysis
化学-应用化学
CiteScore
9.40
自引率
7.40%
发文量
447
审稿时长
1.8 months
期刊介绍:
Advanced Synthesis & Catalysis (ASC) is the leading primary journal in organic, organometallic, and applied chemistry.
The high impact of ASC can be attributed to the unique focus of the journal, which publishes exciting new results from academic and industrial labs on efficient, practical, and environmentally friendly organic synthesis. While homogeneous, heterogeneous, organic, and enzyme catalysis are key technologies to achieve green synthesis, significant contributions to the same goal by synthesis design, reaction techniques, flow chemistry, and continuous processing, multiphase catalysis, green solvents, catalyst immobilization, and recycling, separation science, and process development are also featured in ASC. The Aims and Scope can be found in the Notice to Authors or on the first page of the table of contents in every issue.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


