Synthesis of model heterojunction interfaces reveals molecular-configuration-dependent photoinduced charge transfer
IF 19.2
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
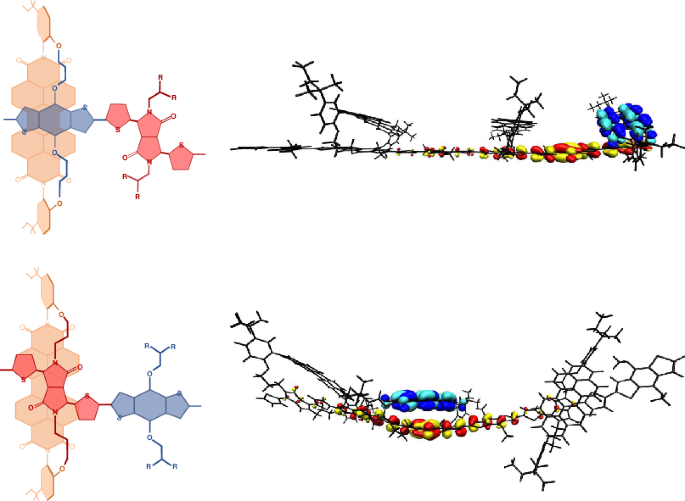
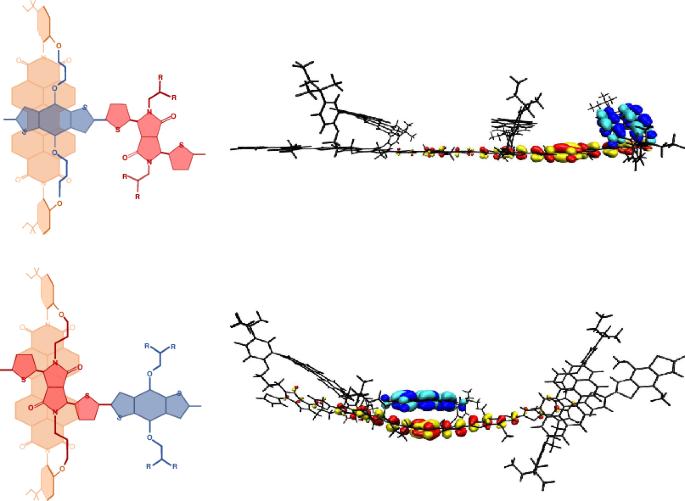
Control of the molecular configuration at the interface of an organic heterojunction is key to the development of efficient optoelectronic devices. Due to the difficulty in characterizing these buried and (probably) disordered heterointerfaces, the interfacial structure in most systems remains a mystery. Here we demonstrate a synthetic strategy to design and control model interfaces, enabling their detailed study in isolation from the bulk material. This is achieved by the synthesis of a polymer in which a non-fullerene acceptor moiety is covalently bonded to a donor polymer backbone using dual alkyl chain links, constraining the acceptor and donor units in a through space co-facial arrangement. The constrained geometry of the acceptor relative to the electron-rich and -poor moieties in the polymer backbone can be tuned to control the kinetics of charge separation and the energy of the resultant charge-transfer state giving insight into factors that govern charge generation at organic heterojunctions. The molecular interactions at an organic heterointerface govern the performance of many optoelectronic devices. Through a combination of synthesis, spectroscopy and modelling, it has now been shown that specific molecular interactions result in improved formation of the charge-transfer state.
模型异质结界面的合成揭示了依赖分子构型的光诱导电荷转移
控制有机异质结界面的分子构型是开发高效光电设备的关键。由于难以表征这些埋藏的(可能)无序异质界面,大多数系统中的界面结构仍然是一个谜。在这里,我们展示了一种设计和控制模型界面的合成策略,从而能够脱离块体材料对其进行详细研究。这是通过合成一种聚合物来实现的,在这种聚合物中,非富勒烯受体分子通过双烷基链键与供体聚合物骨架共价键合,从而将受体和供体单元限制在通孔空间共面排列中。可以调整受体相对于聚合物主链中富电子和贫电子分子的受限几何形状,以控制电荷分离的动力学和由此产生的电荷转移状态的能量,从而深入了解影响有机异质结电荷产生的各种因素。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature chemistry
化学-化学综合
CiteScore
29.60
自引率
1.40%
发文量
226
审稿时长
1.7 months
期刊介绍:
Nature Chemistry is a monthly journal that publishes groundbreaking and significant research in all areas of chemistry. It covers traditional subjects such as analytical, inorganic, organic, and physical chemistry, as well as a wide range of other topics including catalysis, computational and theoretical chemistry, and environmental chemistry.
The journal also features interdisciplinary research at the interface of chemistry with biology, materials science, nanotechnology, and physics. Manuscripts detailing such multidisciplinary work are encouraged, as long as the central theme pertains to chemistry.
Aside from primary research, Nature Chemistry publishes review articles, news and views, research highlights from other journals, commentaries, book reviews, correspondence, and analysis of the broader chemical landscape. It also addresses crucial issues related to education, funding, policy, intellectual property, and the societal impact of chemistry.
Nature Chemistry is dedicated to ensuring the highest standards of original research through a fair and rigorous review process. It offers authors maximum visibility for their papers, access to a broad readership, exceptional copy editing and production standards, rapid publication, and independence from academic societies and other vested interests.
Overall, Nature Chemistry aims to be the authoritative voice of the global chemical community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


