Gut epithelial electrical cues drive differential localization of enterobacteria
IF 20.5
1区 生物学
Q1 MICROBIOLOGY
引用次数: 0
Abstract
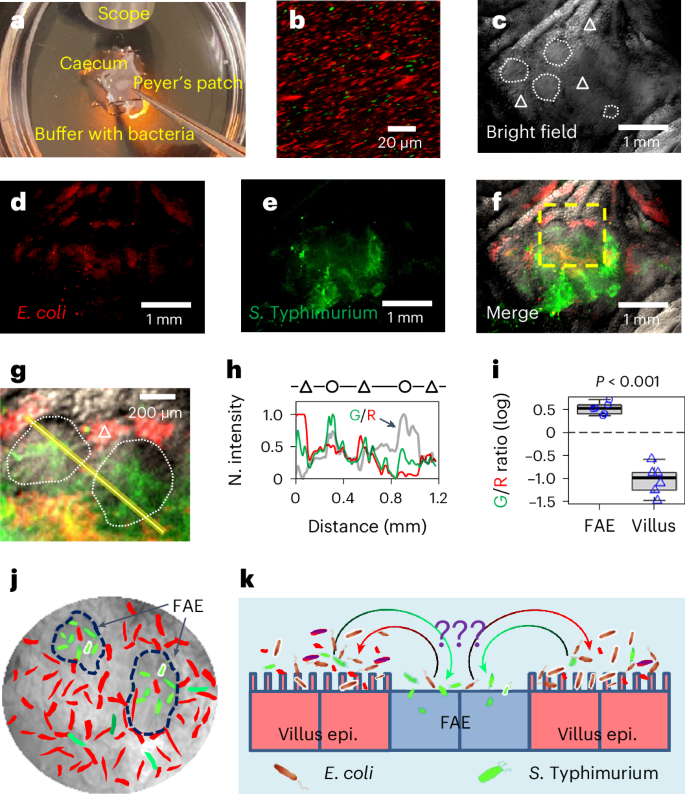
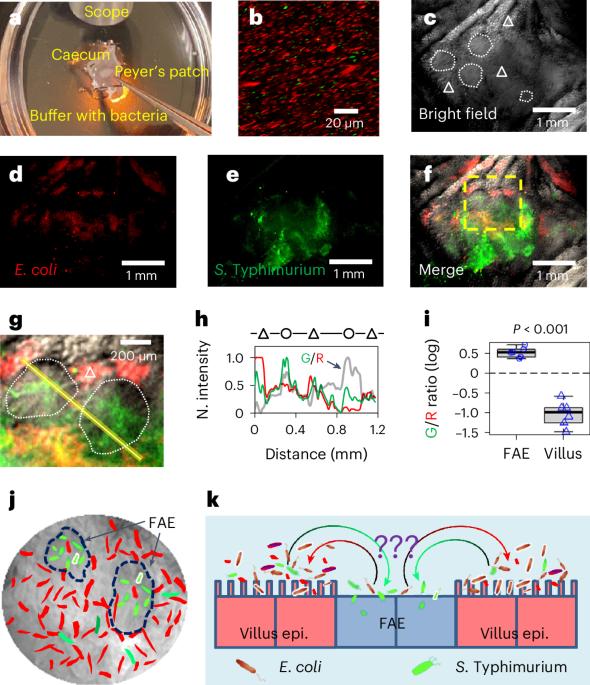
Salmonella translocate to the gut epithelium via microfold cells lining the follicle-associated epithelium (FAE). How Salmonella localize to the FAE is not well characterized. Here we use live imaging and competitive assays between wild-type and chemotaxis-deficient mutants to show that Salmonella enterica serotype Typhimurium (S. Typhimurium) localize to the FAE independently of chemotaxis in an ex vivo mouse caecum infection model. Electrical recordings revealed polarized FAE with sustained outward current and small transepithelial potential, while the surrounding villus is depolarized with inward current and large transepithelial potential. The distinct electrical potentials attracted S. Typhimurium to the FAE while Escherichia coli (E. coli) localized to the villi, through a process called galvanotaxis. Chloride flux involving the cystic fibrosis transmembrane conductance regulator (CFTR) generated the ionic currents around the FAE. Pharmacological inhibition of CFTR decreased S. Typhimurium FAE localization but increased E. coli recruitment. Altogether, our findings demonstrate that bioelectric cues contribute to S. Typhimurium targeting of specific gut epithelial locations, with potential implications for other enteric bacterial infections. Gut epithelium generated electrical potentials drive differential localization of enterobacteria, promoting Salmonella tropism for the follicle-associated epithelium while Escherichia coli localize to villi.
肠道上皮电线索驱动肠杆菌的差异定位
沙门氏菌通过内衬滤泡相关上皮(FAE)的微折叠细胞转运到肠道上皮。沙门氏菌如何定位到 FAE 尚不清楚。在这里,我们利用活体成像和野生型与趋化性缺陷突变体之间的竞争性试验,证明在小鼠盲肠感染模型中,肠炎血清型鼠伤寒沙门氏菌(S. Typhimurium)能独立于趋化性定位到 FAE。电记录显示,极化的FAE具有持续的外向电流和较小的跨上皮电位,而周围的绒毛则是去极化的,具有内向电流和较大的跨上皮电位。不同的电位会吸引伤寒杆菌进入FAE,而大肠埃希氏菌(E. coli)则会通过一种叫做 "电泳 "的过程进入绒毛。涉及囊性纤维化跨膜传导调节因子(CFTR)的氯离子通量在FAE周围产生离子电流。对 CFTR 的药物抑制减少了伤寒杆菌 FAE 的定位,但增加了大肠杆菌的招募。总之,我们的研究结果表明,生物电线索有助于伤寒杆菌锁定特定的肠道上皮位置,这对其他肠道细菌感染具有潜在的影响。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Microbiology
Immunology and Microbiology-Microbiology
CiteScore
44.40
自引率
1.10%
发文量
226
期刊介绍:
Nature Microbiology aims to cover a comprehensive range of topics related to microorganisms. This includes:
Evolution: The journal is interested in exploring the evolutionary aspects of microorganisms. This may include research on their genetic diversity, adaptation, and speciation over time.
Physiology and cell biology: Nature Microbiology seeks to understand the functions and characteristics of microorganisms at the cellular and physiological levels. This may involve studying their metabolism, growth patterns, and cellular processes.
Interactions: The journal focuses on the interactions microorganisms have with each other, as well as their interactions with hosts or the environment. This encompasses investigations into microbial communities, symbiotic relationships, and microbial responses to different environments.
Societal significance: Nature Microbiology recognizes the societal impact of microorganisms and welcomes studies that explore their practical applications. This may include research on microbial diseases, biotechnology, or environmental remediation.
In summary, Nature Microbiology is interested in research related to the evolution, physiology and cell biology of microorganisms, their interactions, and their societal relevance.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


