Distinct active zone protein machineries mediate Ca2+ channel clustering and vesicle priming at hippocampal synapses
IF 21.2
1区 医学
Q1 NEUROSCIENCES
引用次数: 0
Abstract
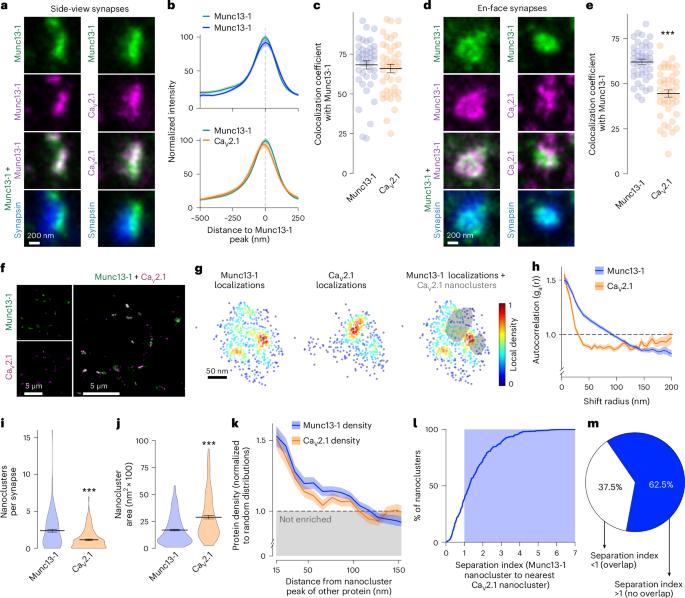
Action potentials trigger neurotransmitter release at the presynaptic active zone with spatiotemporal precision. This is supported by protein machinery that mediates synaptic vesicle priming and clustering of CaV2 Ca2+ channels nearby. One model posits that scaffolding proteins directly tether vesicles to CaV2s; however, here we find that at mouse hippocampal synapses, CaV2 clustering and vesicle priming are executed by separate machineries. CaV2 nanoclusters are positioned at variable distances from those of the priming protein Munc13. The active zone organizer RIM anchors both proteins but distinct interaction motifs independently execute these functions. In transfected cells, Liprin-α and RIM form co-assemblies that are separate from CaV2-organizing complexes. At synapses, Liprin-α1–Liprin-α4 knockout impairs vesicle priming but not CaV2 clustering. The cell adhesion protein PTPσ recruits Liprin-α, RIM and Munc13 into priming complexes without co-clustering CaV2s. We conclude that active zones consist of distinct machineries to organize CaV2s and prime vesicles, and Liprin-α and PTPσ specifically support priming site assembly. The active zone primes synaptic vesicles and clusters voltage-gated Ca2+ channels fast neurotransmitter release. Here the authors dissect the underlying molecular architecture and show that distinct protein machineries execute these functions.
不同的活性区蛋白机制介导海马突触的 Ca2+ 通道集群和囊泡启动
动作电位在突触前活动区触发神经递质的释放,具有时空精确性。这得到了介导突触囊泡启动和附近 CaV2 Ca2+ 通道聚集的蛋白质机制的支持。有一种模型认为,支架蛋白直接将囊泡拴系到 CaV2 上;然而,我们在这里发现,在小鼠海马突触中,CaV2 的聚集和囊泡的启动是由不同的机制执行的。CaV2 纳米簇与引物蛋白 Munc13 纳米簇的位置距离各不相同。活性区组织者 RIM 同时锚定这两种蛋白,但不同的相互作用基序独立执行这些功能。在转染细胞中,Liprin-α 和 RIM 形成了与 CaV2-组织复合物分开的共同集合体。在突触处,Liprin-α1-Liprin-α4 基因敲除会影响囊泡的启动,但不会影响 CaV2 的聚集。细胞粘附蛋白 PTPσ 将 Liprin-α、RIM 和 Munc13 募集到引物复合物中,但不会共同聚集 CaV2。我们的结论是,活性区由不同的机制组成,以组织 CaV2s 和引物囊泡,而 Liprin-α 和 PTPσ 特别支持引物位点的组装。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature neuroscience
医学-神经科学
CiteScore
38.60
自引率
1.20%
发文量
212
审稿时长
1 months
期刊介绍:
Nature Neuroscience, a multidisciplinary journal, publishes papers of the utmost quality and significance across all realms of neuroscience. The editors welcome contributions spanning molecular, cellular, systems, and cognitive neuroscience, along with psychophysics, computational modeling, and nervous system disorders. While no area is off-limits, studies offering fundamental insights into nervous system function receive priority.
The journal offers high visibility to both readers and authors, fostering interdisciplinary communication and accessibility to a broad audience. It maintains high standards of copy editing and production, rigorous peer review, rapid publication, and operates independently from academic societies and other vested interests.
In addition to primary research, Nature Neuroscience features news and views, reviews, editorials, commentaries, perspectives, book reviews, and correspondence, aiming to serve as the voice of the global neuroscience community.
文献相关原料
| 公司名称 | 产品信息 | 采购帮参考价格 |
|---|
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


