Applications of cell therapy in the treatment of virus-associated cancers
IF 81.1
1区 医学
Q1 ONCOLOGY
引用次数: 0
Abstract
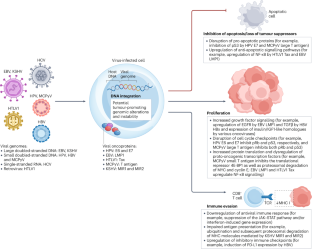
A diverse range of viruses have well-established roles as the primary driver of oncogenesis in various haematological malignancies and solid tumours. Indeed, estimates suggest that approximately 1.5 million patients annually are diagnosed with virus-related cancers. The predominant human oncoviruses include Epstein–Barr virus (EBV), Kaposi sarcoma-associated herpesvirus (KSHV), hepatitis B and C viruses (HBV and HCV), human papillomavirus (HPV), human T-lymphotropic virus type 1 (HTLV1), and Merkel cell polyomavirus (MCPyV). In addition, although not inherently oncogenic, human immunodeficiency virus (HIV) is associated with immunosuppression that contributes to the development of AIDS-defining cancers (specifically, Kaposi sarcoma, aggressive B cell non-Hodgkin lymphoma and cervical cancer). Given that an adaptive T cell-mediated immune response is crucial for the control of viral infections, increasing research is being focused on evaluating virus-specific T cell therapies for the treatment of virus-associated cancers. In this Review, we briefly outline the roles of viruses in the pathogenesis of these malignancies before describing progress to date in the field of virus-specific T cell therapy and evaluating the potential utility of these therapies to treat or possibly even prevent virus-related malignancies. Several different viruses have a role in cancer pathogenesis, contributing to the development of various haematological malignancies and solid tumours via diverse, multifaceted mechanisms. However, this viral aetiology presents a unique opportunity for adoptive virus-specific T cell (VST) therapy. This Review summarizes the mechanisms of viral carcinogenesis and describes the current clinical experience with adoptive cellular immunotherapies for virus-related cancers, predominantly using non-genetically modified VSTs. The authors also discuss challenges and future directions for the ongoing clinical development of VST therapies.

细胞疗法在治疗病毒相关癌症中的应用
在各种血液恶性肿瘤和实体瘤中,各种病毒作为肿瘤发生的主要驱动因素,其作用已得到充分证实。事实上,据估计,每年约有 150 万患者被诊断出患有与病毒相关的癌症。主要的人类肿瘤病毒包括 Epstein-Barr 病毒(EBV)、卡波西肉瘤相关疱疹病毒(KSHV)、乙型肝炎病毒和丙型肝炎病毒(HBV 和 HCV)、人类乳头瘤病毒(HPV)、人类 T 淋巴细胞病毒 1 型(HTLV1)和梅克尔细胞多瘤病毒(MCPyV)。此外,虽然人类免疫缺陷病毒(HIV)本身并不致癌,但它与免疫抑制有关,而免疫抑制会导致艾滋病定义癌症(特别是卡波西肉瘤、侵袭性 B 细胞非霍奇金淋巴瘤和宫颈癌)的发生。鉴于适应性 T 细胞介导的免疫反应对控制病毒感染至关重要,越来越多的研究正集中于评估治疗病毒相关癌症的病毒特异性 T 细胞疗法。在本综述中,我们将简要概述病毒在这些恶性肿瘤发病机制中的作用,然后介绍迄今为止病毒特异性 T 细胞疗法领域取得的进展,并评估这些疗法在治疗甚至可能预防病毒相关恶性肿瘤方面的潜在作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
99.40
自引率
0.40%
发文量
114
审稿时长
6-12 weeks
期刊介绍:
Nature Reviews publishes clinical content authored by internationally renowned clinical academics and researchers, catering to readers in the medical sciences at postgraduate levels and beyond. Although targeted at practicing doctors, researchers, and academics within specific specialties, the aim is to ensure accessibility for readers across various medical disciplines. The journal features in-depth Reviews offering authoritative and current information, contextualizing topics within the history and development of a field. Perspectives, News & Views articles, and the Research Highlights section provide topical discussions, opinions, and filtered primary research from diverse medical journals.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


