Compression-dependent microtubule reinforcement enables cells to navigate confined environments
IF 17.3
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
Abstract
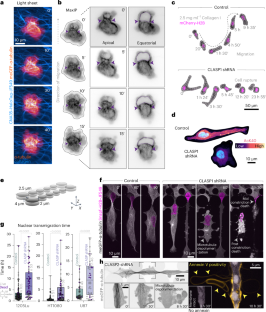
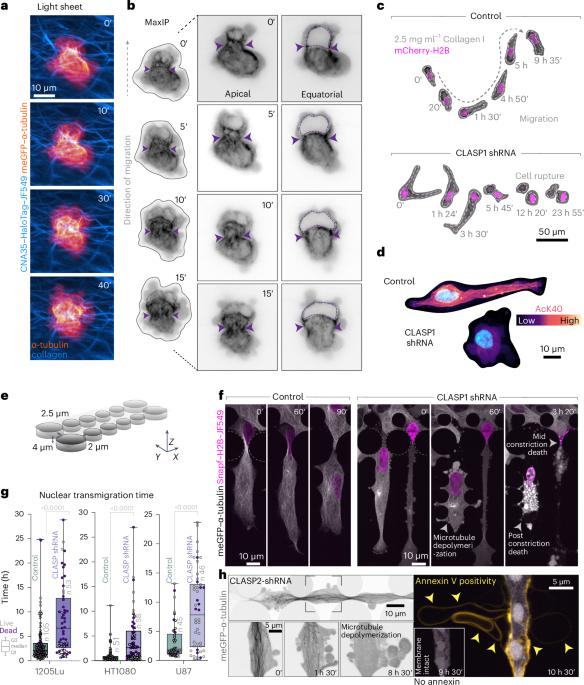
Cells migrating through complex three-dimensional environments experience considerable physical challenges, including tensile stress and compression. To move, cells need to resist these forces while also squeezing the large nucleus through confined spaces. This requires highly coordinated cortical contractility. Microtubules can both resist compressive forces and sequester key actomyosin regulators to ensure appropriate activation of contractile forces. Yet, how these two roles are integrated to achieve nuclear transmigration in three dimensions is largely unknown. Here, we demonstrate that compression triggers reinforcement of a dedicated microtubule structure at the rear of the nucleus by the mechanoresponsive recruitment of cytoplasmic linker-associated proteins, which dynamically strengthens and repairs the lattice. These reinforced microtubules form the mechanostat: an adaptive feedback mechanism that allows the cell to both withstand compressive force and spatiotemporally organize contractility signalling pathways. The microtubule mechanostat facilitates nuclear positioning and coordinates force production to enable the cell to pass through constrictions. Disruption of the mechanostat imbalances cortical contractility, stalling migration and ultimately resulting in catastrophic cell rupture. Our findings reveal a role for microtubules as cellular sensors that detect and respond to compressive forces, enabling movement and ensuring survival in mechanically demanding environments. Ju et al. show that during three-dimensional cell migration, compression recruits cytoplasmic linker-associated proteins to microtubules; these stabilized microtubules then coordinate nuclear positioning and contractility in confined migration.
依赖压缩的微管强化使细胞能够在密闭环境中航行
细胞在复杂的三维环境中迁移时会遇到相当大的物理挑战,包括拉伸应力和压缩力。为了移动,细胞需要抵抗这些力,同时还要挤压庞大的细胞核穿过狭窄的空间。这需要高度协调的皮质收缩力。微管既能抵抗压缩力,又能封闭关键的肌动蛋白调节因子,以确保适当激活收缩力。然而,如何整合这两种作用以实现核在三维空间中的迁移在很大程度上还是未知数。在这里,我们证明了压缩会触发细胞核后部的专用微管结构的加固,这种加固是通过细胞质连接体相关蛋白的机械响应招募来实现的,从而动态地加固和修复晶格。这些加固的微管构成了机械抑制器:一种适应性反馈机制,使细胞既能承受压缩力,又能在时空上组织收缩信号通路。微管机械静止器有助于细胞核定位并协调力的产生,使细胞能够通过收缩。机械促进剂的破坏会导致皮质收缩力失衡、迁移停滞并最终导致细胞灾难性破裂。我们的研究结果揭示了微管作为细胞传感器的作用,它能检测压缩力并做出反应,从而使细胞运动起来,并确保细胞在机械要求极高的环境中存活。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Cell Biology
生物-细胞生物学
CiteScore
28.40
自引率
0.90%
发文量
219
审稿时长
3 months
期刊介绍:
Nature Cell Biology, a prestigious journal, upholds a commitment to publishing papers of the highest quality across all areas of cell biology, with a particular focus on elucidating mechanisms underlying fundamental cell biological processes. The journal's broad scope encompasses various areas of interest, including but not limited to:
-Autophagy
-Cancer biology
-Cell adhesion and migration
-Cell cycle and growth
-Cell death
-Chromatin and epigenetics
-Cytoskeletal dynamics
-Developmental biology
-DNA replication and repair
-Mechanisms of human disease
-Mechanobiology
-Membrane traffic and dynamics
-Metabolism
-Nuclear organization and dynamics
-Organelle biology
-Proteolysis and quality control
-RNA biology
-Signal transduction
-Stem cell biology
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


