Prognostic impact of shift to low visceral fat mass after neoadjuvant chemotherapy in patients with esophageal cancer
Abstract
Background
Based on the JCOG1109 trial, it is suggested that the combination of docetaxel, cisplatin, and 5-fluorouracil (DCF) could potentially become a standard neoadjuvant chemotherapy regimen, alongside the conventional 5-fluorouracil and cisplatin (CF) therapy, for esophageal cancer. However, there are few reports on the impact of body composition changes associated with neoadjuvant chemotherapy on prognosis.
Aim
Our study aimed to explore the effect of different neoadjuvant chemotherapy regimens on body composition during treatment and the impacts of body composition changes on their prognosis.
Methods and results
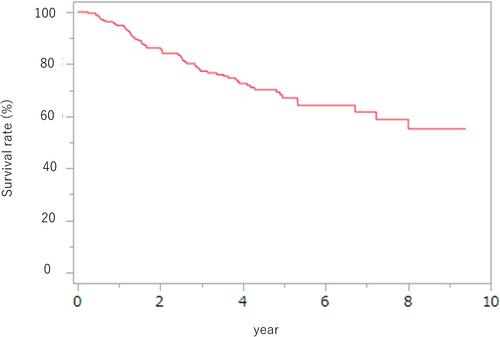
This is a retrospective study of 215 patients with advanced thoracic esophageal cancer who had surgery after neoadjuvant chemotherapy from 2013 to 2019. Computed tomography scans were performed before and after neoadjuvant chemotherapy to assess body composition. Skeletal muscle mass index (SMI) was calculated by dividing total skeletal muscle mass at the 3rd lumbar level by the square of height, while visceral and subcutaneous fat masses were measured at the level of umbilicus. Patients in the lowest 25% of both sexes were classified into the low visceral fat and low subcutaneous fat groups, respectively. Of the patients enrolled, 178 were male and 37 were female. Among them, 91 had clinical Stage II disease, and 124 had clinical Stage III disease. Additionally, 146 patients received neoadjuvant chemotherapy CF, and 69 received neoadjuvant chemotherapy DCF. Comparing the DCF and CF groups, the DCF group consisted of significantly younger patients (p < .01), a higher proportion of males (p = .03), and a greater number of clinical Stage III cases (p < .01). However, although percent change in SMI and visceral fat mass was not significantly different between two regimens, percent change in subcutaneous fat mass was significant in the DCF group. The major prognostic factors for patients undergoing surgery after neoadjuvant chemotherapy for thoracic esophageal cancer were clinical Stage III, transition to low visceral fat, and response rating (SD/PD), while the specific neoadjuvant chemotherapy regimen did not significantly influence the outcomes.
Conclusion
This study suggests that prevention of the shift to low visceral fat throughout the neoadjuvant chemotherapy process should improve patient outcomes.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


