Copper-catalyzed intermolecular formal [4 + 1] annulation of 1,5-diynes with benzocyclobutenones
引用次数: 0
Abstract
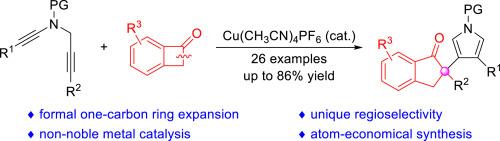
The one-carbon ring expansion of benzocyclobutenones is an efficient methodology for the assembly of cyclic ketones. However, hazardous reagents or noble metal catalysts are frequently required. Herein, we disclose a copper-catalyzed intermolecular formal [4 + 1] annulation of 1,5-diynes with benzocyclobutenones, allowing the practical and atom-economical construction of diverse pyrryl 1-indanones in generally good yields under mild reaction conditions. This reaction represents an important advancement in the ring expansion of benzocyclobutenones via vinyl cation pathway.
铜催化 1,5-二炔与苯并环丁烯酮的分子间正规 [4 + 1] 环化反应
苯并环丁烯酮的一碳环扩展是组装环酮的有效方法。然而,这种方法经常需要使用危险试剂或贵金属催化剂。在此,我们揭示了一种铜催化的 1,5- 二炔与苯并环丁烯酮的分子间形式 [4 + 1] 环化反应,从而可以在温和的反应条件下,以普遍较好的产率,构建出实用且原子经济的各种吡咯 1-茚酮。该反应是苯并环丁烯酮通过乙烯基阳离子途径扩环的重要进展。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


