A fibroblast activation protein α-activatable nanoagent co-delivering diethyldithiocarbamate and copper for tumor therapy and imaging
IF 9.4
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
Disulfiram (DSF), an FDA-approved drug for treating alcoholism, has been verified with Cu2+-dependent anticancer activity by forming Cu(DTC)2, the complex of one of its metabolites diethyldithiocarbamate (DTC) and Cu2+. Nevertheless, the antitumor effect is limited by insufficient Cu(DTC)2 formation in suit and off-target system toxicity. Herein, we developed a fibroblast activation protein α (FAPα) activatable nanoagent (HfD-HID-Cu) for co-delivery of DTC polymeric prodrug and exogenous Cu2+ to achieve enhanced cancer-specific therapy and activatable in situ fluorescence imaging meanwhile. HfD-HID-Cu was simply constructed through the co-assembly of the DTC polymeric prodrug (HA-fap-DTC) and the copper-loaded IR808-conjugated polymer (HA-IR-DPA-Cu), which could serve as the “OFF-to-ON” switch for chemotherapy and fluorescence. With the high expression of FAPα in tumor tissues, HA-fap-DTC could be activated specifically to release DTC, while maintaining inactive in normal tissues. The liberated DTC within tumor tissues could contend for Cu2+ from HA-IR-DPA-Cu, resulting in the formation of highly cytotoxic Cu(DTC)2 in situ for chemotherapy, concomitant with the fluorescence recovery of cyanine dye for tumor imaging. This work provides an effective strategy for co-delivery of DTC prodrug and Cu2+ for tumor theranostic with improved selectivity and minimal side effects.
Statement of significance
DSF-based antitumor therapy is highly dependent on Cu2+. However, the non-synchronous distribution of DSF/DTC and Cu2+ in tumor tissues attenuates the antitumor efficacy. The insufficient Cu(DTC)2 formation in suit and off-target distribution greatly limit the anti-tumor application. This study provides a nanoagent for co-delivery of DTC polymeric prodrug and Cu2+ by simple co-assembly to achieve their synchronous tumor distribution. It can be selectively activated by FAPα, forming cytotoxic Cu(DTC)2 in suit for tumor-specific chemotherapy and reducing the systemic toxicity. In addition to chemotherapy, the nanoagent can emit fluorescence under the sequential triggering of FAPα and released DTC for tumor imaging. Overall, this study renders a promising strategy for improved Cu(DTC)2-based antitumor therapy and imaging.
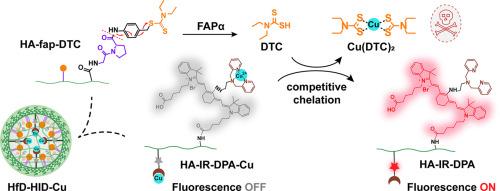
一种可激活成纤维细胞活化蛋白α的纳米试剂,可同时释放二乙基二硫代氨基甲酸乙酯和铜,用于肿瘤治疗和成像。
二硫代二硫代氨基甲酸二乙酯(DTC)与 Cu2+ 形成的复合物 Cu(DTC)2 具有 Cu2+ 依赖性抗癌活性。然而,由于 Cu(DTC)2 在服药过程中形成不足以及脱靶系统毒性,其抗肿瘤效果受到了限制。在此,我们开发了一种成纤维细胞活化蛋白α(FAPα)可活化纳米试剂(HfD-HID-Cu),用于DTC聚合物原药和外源Cu2+的联合给药,以实现增强的癌症特异性治疗,同时可活化原位荧光成像。HfD-HID-Cu由DTC聚合物原药(HA-fap-DTC)和铜负载的IR808共轭聚合物(HA-IR-DPA-Cu)共同组装而成,可作为化疗和荧光的 "OFF-to-ON "开关。由于 FAPα 在肿瘤组织中的高表达,HA-fap-DTC 可被特异性激活以释放 DTC,而在正常组织中则保持非活性。在肿瘤组织中释放的 DTC 可与 HA-IR-DPA-Cu 中的 Cu2+ 竞争,从而在原位形成高细胞毒性的 Cu(DTC)2 用于化疗,同时还可恢复青色染料的荧光用于肿瘤成像。这项研究为将 DTC 原药和 Cu2+ 联合递送用于肿瘤治疗提供了一种有效的策略,具有更高的选择性和最小的副作用。意义说明:基于 DSF 的抗肿瘤疗法高度依赖 Cu2+。然而,DSF/DTC 和 Cu2+ 在肿瘤组织中的非同步分布削弱了抗肿瘤疗效。Cu(DTC)2在服药过程中形成不足以及脱靶分布极大地限制了抗肿瘤药物的应用。本研究通过简单的共组装,提供了一种 DTC 高分子原药和 Cu2+ 共同给药的纳米试剂,实现了它们在肿瘤中的同步分布。它可被 FAPα 选择性激活,形成具有细胞毒性的 Cu(DTC)2 适合肿瘤特异性化疗,并降低全身毒性。除化疗外,该纳米试剂还能在 FAPα 和释放的 DTC 相继触发下发出荧光,用于肿瘤成像。总之,这项研究为改进基于 Cu(DTC)2 的抗肿瘤治疗和成像提供了一种前景广阔的策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Acta Biomaterialia
工程技术-材料科学:生物材料
CiteScore
16.80
自引率
3.10%
发文量
776
审稿时长
30 days
期刊介绍:
Acta Biomaterialia is a monthly peer-reviewed scientific journal published by Elsevier. The journal was established in January 2005. The editor-in-chief is W.R. Wagner (University of Pittsburgh). The journal covers research in biomaterials science, including the interrelationship of biomaterial structure and function from macroscale to nanoscale. Topical coverage includes biomedical and biocompatible materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


