Synthesis of 2‐Substituted Bicyclo[2.1.1]Hexan‐1‐ols via SmI2‐Mediated Reductive Cyclization Reactions
IF 4.4
2区 化学
Q2 CHEMISTRY, APPLIED
引用次数: 0
Abstract
The replacement of benzene rings with saturated bioisosteric counterparts is a key priority in drug discovery programs, and disubstituted bicyclo[2.1.1]hexanes have been recognized as flexible molecular scaffolds that could act as ortho‐substituted benzene bioisosteres. In this study, we outline the synthesis of a wide range of 2‐substituted bicyclo[2.1.1]hexan‐1‐ols, which have the potential to emulate ortho‐phenolic derivatives, via SmI2‐mediated reductive cyclization reactions. The synthetic utility of this methodology was exemplified by the preparation of several saturated analogs of pharmaceutically relevant compounds.
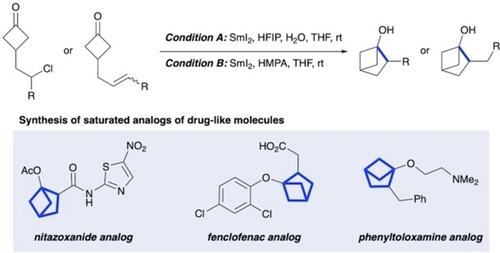
通过 SmI2 介导的还原环化反应合成 2-取代的双环[2.1.1]己-1-醇
用饱和的生物异斯特对应物取代苯环是药物发现计划中的一个关键优先事项,而二取代的双环[2.1.1]己烷已被认为是可用作<i>正交</i>取代苯生物异斯特的灵活分子支架。在本研究中,我们概述了通过 SmI<sub>2</sub>介导的还原环化反应合成多种 2-取代的双环[2.1.1]己-1-醇的过程,这些醇具有仿效 <i>正交</i>-酚类衍生物的潜力。这种方法的合成实用性体现在制备了几种药用相关化合物的饱和类似物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Advanced Synthesis & Catalysis
化学-应用化学
CiteScore
9.40
自引率
7.40%
发文量
447
审稿时长
1.8 months
期刊介绍:
Advanced Synthesis & Catalysis (ASC) is the leading primary journal in organic, organometallic, and applied chemistry.
The high impact of ASC can be attributed to the unique focus of the journal, which publishes exciting new results from academic and industrial labs on efficient, practical, and environmentally friendly organic synthesis. While homogeneous, heterogeneous, organic, and enzyme catalysis are key technologies to achieve green synthesis, significant contributions to the same goal by synthesis design, reaction techniques, flow chemistry, and continuous processing, multiphase catalysis, green solvents, catalyst immobilization, and recycling, separation science, and process development are also featured in ASC. The Aims and Scope can be found in the Notice to Authors or on the first page of the table of contents in every issue.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


