Diversified Reactivity of Triphenylphosphine: Reinvestigation of the Phosphine-Mediated Reductive Condensation Approach for the Synthesis of Substituted Furans
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
A reinvestigation of “Phosphine-Mediated Reductive Condensation of γ-Acyloxy Butynoates: A Diversity Oriented Strategy for the Construction of Substituted Furans” (J. Am. Chem. Soc. 2004, 126, 4118–4119) revealed different chemoselectivity of triphenylphosphine in the reactions with the γ-acyloxy butynoate substrates of varying substitution patterns/electronics. Furthermore, the electronics of the triaryl phosphine reagent could be tuned to trap a putative intermediate such as A, leading to the semihydrogenation of propiolamide substrates.
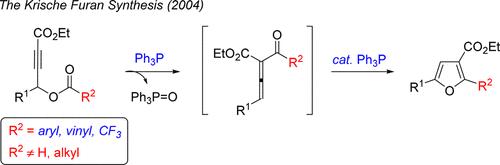
三苯基膦的多样化反应活性:膦介导的用于合成取代呋喃的还原缩合方法的再研究
对 "Phosphine-Mediated Reductive Condensation of γ-Acyloxy Butynoates:J. Am. Chem. Soc. 2004, 126, 4118-4119)发现,三苯基膦在与γ-乙酰氧基丁炔酸盐底物的反应中具有不同的化学选择性。此外,还可以调整三芳基膦试剂的电子学特性,以捕获推定的中间体(如 A),从而导致丙炔酰胺底物的半氢化反应。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


