Temperature Self-Limited Intelligent Thermo-chemotherapeutic Lipid Nanosystem for P-gp Reversal Time Window Matched Pulse Treatment of MDR Tumor
IF 9.1
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Mild photothermal therapy (PTT) shows the potential for chemosensitization by tumor-localized P-glycoprotein (P-gp) modulation. However, conventional mild PTT struggles with real-time uniform temperature control, obscuring the temperature–performance relationship and resulting in thermal damage. Besides, the time-performance relationship and the underlying mechanism of mild PTT-mediated P-gp reversal remains elusive. Herein, we developed a temperature self-limiting lipid nanosystem (RFE@PD) that integrated a reversible organic heat generator (metal-phenolic complexes) and metal chelator (deferiprone, DFP) encapsulated phase change material. Upon NIR irradiation, RFE@PD released DFP for blocking ligand–metal charge transfer to self-limit temperature below 45 °C, and rapidly reduced P-gp within 3 h via Ubiquitin-proteasome degradation. Consequently, the DOX·HCl-loaded thermo-chemotherapeutic lipid nanosystem (RFE@PD-DOX) led to dramatically improved drug accumulation and 5-fold chemosensitization in MCF-7/ADR tumor models by synchronizing P-gp reversal and drug pulse liberation, achieving a tumor inhibition ratio of 82.42%. This lipid nanosystem integrated with “intrinsic temperature-control” and “temperature-responsive pulse release” casts new light on MDR tumor therapy.
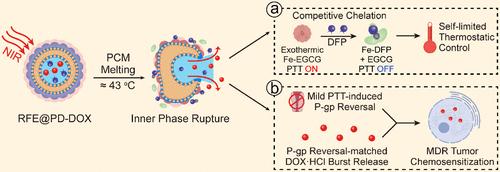
用于 MDR 肿瘤 P-gp 逆转时间窗匹配脉冲治疗的温度自限智能热化学治疗脂质纳米系统
温和光热疗法(PTT)通过调节肿瘤定位的糖蛋白(P-gp)而显示出化疗增敏的潜力。然而,传统的温和光热疗法难以实现实时均匀的温度控制,模糊了温度-性能关系,导致热损伤。此外,温和 PTT 介导的 P-gp 逆转的时间-性能关系和内在机制仍不清楚。在此,我们开发了一种温度自限制脂质纳米系统(RFE@PD),该系统集成了可逆有机发热体(金属酚类复合物)和金属螯合剂(去铁酮,DFP)封装的相变材料。在近红外照射下,RFE@PD 释放出 DFP,用于阻断配体-金属电荷转移,使自我限温低于 45 °C,并在 3 小时内通过泛素-蛋白酶体降解迅速减少 P-gp。因此,负载 DOX-HCl 的热化学治疗脂质纳米系统(RFE@PD-DOX)在 MCF-7/ADR 肿瘤模型中通过同步 P-gp 逆转和药物脉冲释放,显著提高了药物蓄积和 5 倍的化疗敏感性,实现了 82.42% 的肿瘤抑制率。这种集 "内在温度控制 "和 "温度响应脉冲释放 "于一体的脂质纳米系统为 MDR 肿瘤治疗带来了新的曙光。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nano Letters
工程技术-材料科学:综合
CiteScore
16.80
自引率
2.80%
发文量
1182
审稿时长
1.4 months
期刊介绍:
Nano Letters serves as a dynamic platform for promptly disseminating original results in fundamental, applied, and emerging research across all facets of nanoscience and nanotechnology. A pivotal criterion for inclusion within Nano Letters is the convergence of at least two different areas or disciplines, ensuring a rich interdisciplinary scope. The journal is dedicated to fostering exploration in diverse areas, including:
- Experimental and theoretical findings on physical, chemical, and biological phenomena at the nanoscale
- Synthesis, characterization, and processing of organic, inorganic, polymer, and hybrid nanomaterials through physical, chemical, and biological methodologies
- Modeling and simulation of synthetic, assembly, and interaction processes
- Realization of integrated nanostructures and nano-engineered devices exhibiting advanced performance
- Applications of nanoscale materials in living and environmental systems
Nano Letters is committed to advancing and showcasing groundbreaking research that intersects various domains, fostering innovation and collaboration in the ever-evolving field of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


