Structural plasticity of bacterial ESCRT-III protein PspA in higher-order assemblies
IF 12.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
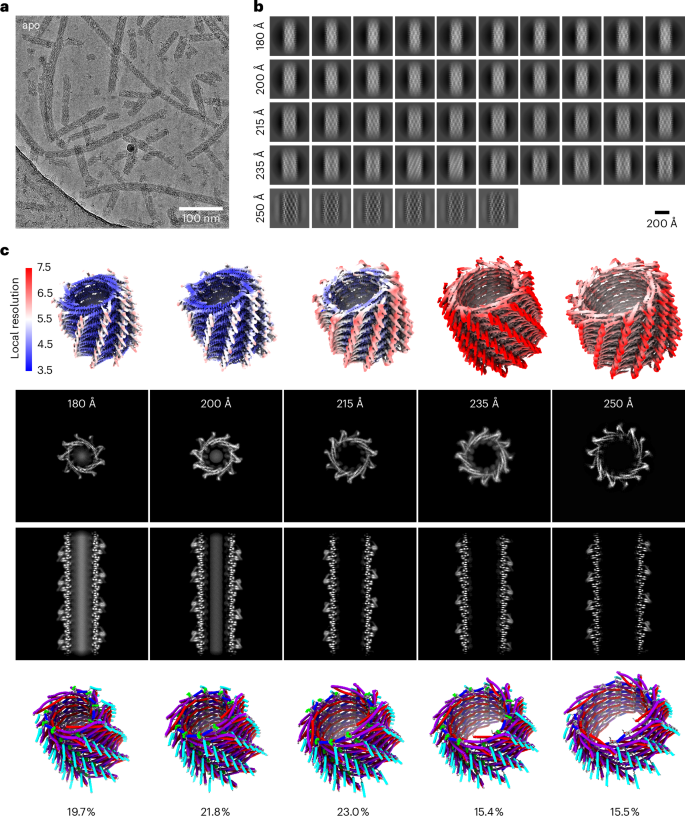
Eukaryotic members of the endosome sorting complex required for transport-III (ESCRT-III) family have been shown to form diverse higher-order assemblies. The bacterial phage shock protein A (PspA) has been identified as a member of the ESCRT-III superfamily, and PspA homo-oligomerizes to form rod-shaped assemblies. As observed for eukaryotic ESCRT-III, PspA forms tubular assemblies of varying diameters. Using electron cryo-electron microscopy, we determined 61 Synechocystis PspA structures and observed in molecular detail how the structural plasticity of PspA rods is mediated by conformational changes at three hinge regions in the monomer and by the fixed and changing molecular contacts between protomers. Moreover, we reduced and increased the structural plasticity of PspA rods by removing the loop connecting helices α3/α4 and the addition of nucleotides, respectively. Based on our analysis of PspA-mediated membrane remodeling, we suggest that the observed mode of structural plasticity is a prerequisite for the biological function of ESCRT-III members. Using cryo-EM, the authors revealed structures of ESCRT-III superfamily member PspA and the molecular basis of structural plasticity that enables assembly modulations by the addition of nucleotides and targeted mutations.

细菌 ESCRT-III 蛋白 PspA 在高阶组装中的结构可塑性
真核生物的内质体分选复合体转运-III(ESCRT-III)家族成员已被证明能形成多种高阶组合。细菌噬菌体休克蛋白 A(PspA)已被确定为 ESCRT-III 超家族的成员,PspA 同源寡聚形成杆状组装。正如在真核生物 ESCRT-III 中观察到的那样,PspA 形成不同直径的管状集合体。我们利用电子低温电镜测定了 Synechocystis PspA 的 61 个结构,并从分子角度详细观察了 PspA 杆状体的结构可塑性是如何通过单体中三个铰链区的构象变化以及原生体之间固定和变化的分子接触来实现的。此外,我们还分别通过去除连接螺旋α3/α4的环和添加核苷酸来减少和增加PspA杆的结构可塑性。根据我们对 PspA 介导的膜重塑的分析,我们认为观察到的结构可塑性模式是 ESCRT-III 成员发挥生物功能的先决条件。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Structural & Molecular Biology
BIOCHEMISTRY & MOLECULAR BIOLOGY-BIOPHYSICS
CiteScore
22.00
自引率
1.80%
发文量
160
审稿时长
3-8 weeks
期刊介绍:
Nature Structural & Molecular Biology is a comprehensive platform that combines structural and molecular research. Our journal focuses on exploring the functional and mechanistic aspects of biological processes, emphasizing how molecular components collaborate to achieve a particular function. While structural data can shed light on these insights, our publication does not require them as a prerequisite.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


