Contrasting Facial Selectivity of a Squaramide‐Tagged Proline in the Asymmetric Michael Addition of Ketones to Maleimides
IF 4.4
2区 化学
Q2 CHEMISTRY, APPLIED
引用次数: 0
Abstract
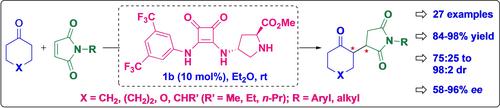
A squaramide moiety was introduced at the C‐4 position of proline to afford an organocatalyst that served as a stereocontrol element to promote the asymmetric Michael addition of cyclic ketones to maleimides. A variety of chiral succinimides were obtained by the conjugate addition in 84–98% yield accompanied by 58–96% enantioselectivity. The results also include an interesting contrast in the facial selectivity observed with cyclohexanones and cycloheptanones, whereas an asymmetric desymmetrization of 4‐alkyl cyclohexanones was also achieved using the transformation.
Squaramide 标记的脯氨酸在酮与马来酰亚胺的不对称迈克尔加成中的对比面选择性
在脯氨酸的 C-4 位引入了一个方酰胺分子,从而获得了一种有机催化剂,该催化剂可作为立体控制元件,促进环酮与马来酰亚胺的不对称迈克尔加成反应。通过共轭加成得到了多种手性琥珀酰亚胺,产率为 84-98%,对映选择性为 58-96%。研究结果还发现,环己酮和环庚酮的面选择性形成了有趣的对比,而 4-烷基环己酮的不对称非对称化也是通过这种转化实现的。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Advanced Synthesis & Catalysis
化学-应用化学
CiteScore
9.40
自引率
7.40%
发文量
447
审稿时长
1.8 months
期刊介绍:
Advanced Synthesis & Catalysis (ASC) is the leading primary journal in organic, organometallic, and applied chemistry.
The high impact of ASC can be attributed to the unique focus of the journal, which publishes exciting new results from academic and industrial labs on efficient, practical, and environmentally friendly organic synthesis. While homogeneous, heterogeneous, organic, and enzyme catalysis are key technologies to achieve green synthesis, significant contributions to the same goal by synthesis design, reaction techniques, flow chemistry, and continuous processing, multiphase catalysis, green solvents, catalyst immobilization, and recycling, separation science, and process development are also featured in ASC. The Aims and Scope can be found in the Notice to Authors or on the first page of the table of contents in every issue.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


