Lymphotoxin-β promotes breast cancer bone metastasis colonization and osteolytic outgrowth
IF 17.3
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
Abstract
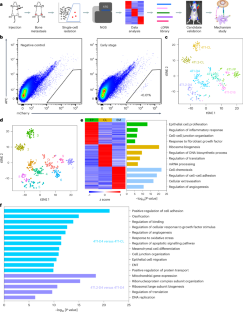
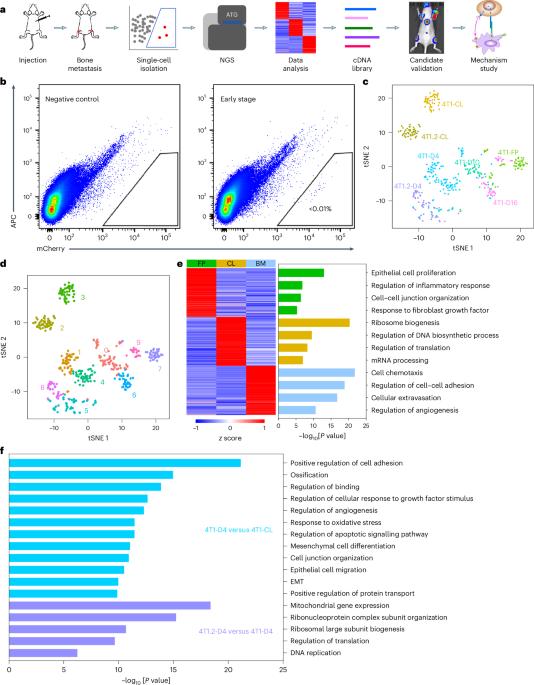
Bone metastasis is a lethal consequence of breast cancer. Here we used single-cell transcriptomics to investigate the molecular mechanisms underlying bone metastasis colonization—the rate-limiting step in the metastatic cascade. We identified that lymphotoxin-β (LTβ) is highly expressed in tumour cells within the bone microenvironment and this expression is associated with poor bone metastasis-free survival. LTβ promotes tumour cell colonization and outgrowth in multiple breast cancer models. Mechanistically, tumour-derived LTβ activates osteoblasts through nuclear factor-κB2 signalling to secrete CCL2/5, which facilitates tumour cell adhesion to osteoblasts and accelerates osteoclastogenesis, leading to bone metastasis progression. Blocking LTβ signalling with a decoy receptor significantly suppressed bone metastasis in vivo, whereas clinical sample analysis revealed significantly higher LTβ expression in bone metastases than in primary tumours. Our findings highlight LTβ as a bone niche-induced factor that promotes tumour cell colonization and osteolytic outgrowth and underscore its potential as a therapeutic target for patients with bone metastatic disease. Wang, Zhang, Zheng et al. demonstrate that tumour cell-derived lymphotoxin-β activates NF-κB2 signalling and CCL2/5 secretion in osteoblasts to promote bone metastasis in breast cancer, which may potentially be targeted with a decoy receptor in vivo.
淋巴毒素-β促进乳腺癌骨转移瘤的定植和溶骨生长
骨转移是乳腺癌的致命后果。在这里,我们利用单细胞转录组学研究了骨转移定植的分子机制--骨转移级联过程中的限速步骤。我们发现,淋巴毒素-β(LTβ)在骨微环境中的肿瘤细胞中高表达,而这种表达与骨转移无生存率低有关。在多种乳腺癌模型中,LTβ能促进肿瘤细胞的定植和生长。从机制上讲,肿瘤衍生的LTβ通过核因子-κB2信号激活成骨细胞分泌CCL2/5,从而促进肿瘤细胞粘附到成骨细胞并加速破骨细胞生成,导致骨转移进展。用诱饵受体阻断LTβ信号可明显抑制体内骨转移,而临床样本分析显示,骨转移瘤中LTβ的表达明显高于原发肿瘤。我们的研究结果突出表明,LTβ是一种骨龛诱导因子,可促进肿瘤细胞定植和溶骨生长,并强调了其作为骨转移患者治疗靶点的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Cell Biology
生物-细胞生物学
CiteScore
28.40
自引率
0.90%
发文量
219
审稿时长
3 months
期刊介绍:
Nature Cell Biology, a prestigious journal, upholds a commitment to publishing papers of the highest quality across all areas of cell biology, with a particular focus on elucidating mechanisms underlying fundamental cell biological processes. The journal's broad scope encompasses various areas of interest, including but not limited to:
-Autophagy
-Cancer biology
-Cell adhesion and migration
-Cell cycle and growth
-Cell death
-Chromatin and epigenetics
-Cytoskeletal dynamics
-Developmental biology
-DNA replication and repair
-Mechanisms of human disease
-Mechanobiology
-Membrane traffic and dynamics
-Metabolism
-Nuclear organization and dynamics
-Organelle biology
-Proteolysis and quality control
-RNA biology
-Signal transduction
-Stem cell biology
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


