Converging deep learning and human-observed tumor-adipocyte interaction as a biomarker in colorectal cancer
IF 5.4
Q1 MEDICINE, RESEARCH & EXPERIMENTAL
引用次数: 0
Abstract
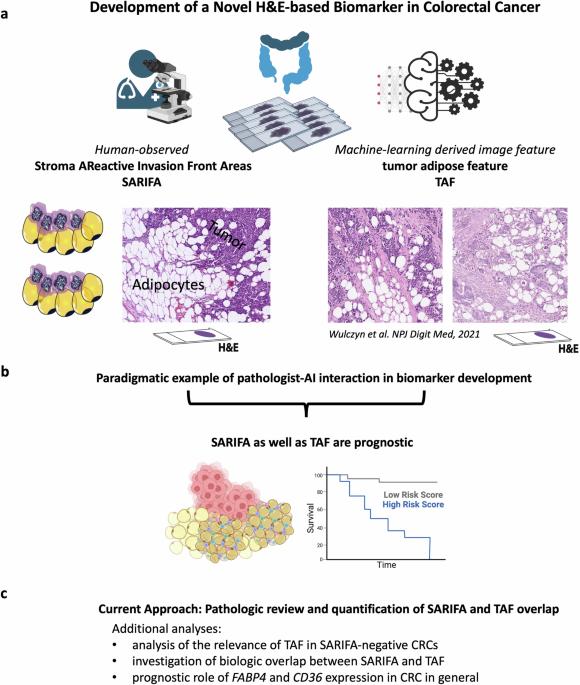
Tumor-Adipose-Feature (TAF) as well as SARIFA (Stroma AReactive Invasion Front Areas) are two histologic features/biomarkers linking tumor-associated adipocytes to poor outcomes in colorectal cancer (CRC) patients. Whereas TAF was identified by deep learning (DL) algorithms, SARIFA was established as a human-observed histopathologic biomarker. To study the overlap between TAF and SARIFA, we performed a systematic pathological review of TAF based on all published image tiles. Additionally, we analyzed the presence/absence of TAF in SARIFA-negative CRC cases to elucidate the biologic and prognostic role of a direct tumor-adipocyte contact. TCGA-CRC gene expression data is investigated to assess the association of FABP4 (fatty-acid binding protein 4) and CD36 (fatty-acid translocase) with both TAF and CRC prognosis. By investigating the TAF/SARIFA overlap, we show that many TAF patches correspond to the recently described SARIFA-phenomenon. Even though there is a pronounced morphological and biological overlap, there are differences in the concepts. The presence of TAF in SARIFA-negative CRCs is not associated with poor outcomes in this cohort, potentially highlighting the importance of a direct tumor-adipocyte interaction. Upregulation of FABP4 and CD36 gene expression seem both linked to a poor prognosis in CRC. By proving the substantial overlap between human-observed SARIFA and DL-based TAF as morphologic biomarkers, we demonstrate that linking DL-based image features to independently developed histopathologic biomarkers is a promising tool in the identification of clinically and biologically meaningful biomarkers. Adipocyte-tumor-cell interactions seem to be crucial in CRC, which should be considered as biomarkers for further investigations. Different methods exist in assessing samples removed from cancer patients during surgery. We linked two independently established tissue-based methods for determining the outcome of colorectal cancer patients together: tumor adipose feature (TAF) and Stroma AReactive Invasion Front Areas (SARIFA). SARIFA as biological feature was observed solely by humans and TAF was identified by the help of a computer algorithm. We examined TAF in many cancer slides and looked at whether they showed similarities to SARIFA. TAF often matched SARIFA, but not always. Interestingly, these methods could be used to predict outcomes for patients and are associated with specific gene expression involved in tumor and fat cell interaction. Our study shows that combining computer algorithms with human expertize in evaluating tissue samples can identify meaningful features in patient samples, which may help to predict the best treatment options. Reitsam et al. assess the overlap between deep learning- and human observer-based identification of tumor-adipocyte interaction as a promising biomarker in colorectal cancer. They demonstrate that combining artificial intelligence and human pathological expertize is beneficial in identifying clinically relevant biomarkers.
融合深度学习和人类观察到的肿瘤与脂肪细胞相互作用作为结直肠癌的生物标记物
肿瘤脂肪特征(TAF)和SARIFA(基质活性侵袭前区)是将肿瘤相关脂肪细胞与结直肠癌(CRC)患者不良预后联系起来的两个组织学特征/生物标记物。TAF是通过深度学习(DL)算法识别的,而SARIFA则是人类观察到的组织病理学生物标记。为了研究 TAF 和 SARIFA 之间的重叠,我们根据所有已发表的图像瓦片对 TAF 进行了系统的病理学审查。此外,我们还分析了 SARIFA 阴性 CRC 病例中 TAF 的存在/不存在情况,以阐明肿瘤与脂肪细胞直接接触的生物和预后作用。我们研究了TCGA-CRC基因表达数据,以评估FABP4(脂肪酸结合蛋白4)和CD36(脂肪酸转运酶)与TAF和CRC预后的关联。通过研究 TAF/SARIFA 重叠,我们发现许多 TAF 斑块与最近描述的 SARIFA 现象相对应。尽管在形态学和生物学上有明显的重叠,但在概念上却存在差异。SARIFA 阴性 CRC 中 TAF 的存在与该队列中的不良预后无关,这可能凸显了肿瘤与脂肪细胞直接相互作用的重要性。FABP4和CD36基因表达的上调似乎都与CRC的不良预后有关。通过证明人类观察到的 SARIFA 与基于 DL 的 TAF 作为形态学生物标志物之间存在大量重叠,我们证明了将基于 DL 的图像特征与独立开发的组织病理学生物标志物联系起来是一种很有前途的工具,可用于鉴定临床和生物学意义的生物标志物。脂肪细胞与肿瘤细胞之间的相互作用似乎在 CRC 中至关重要,应将其视为生物标志物进行进一步研究。评估癌症患者手术中取出的样本有不同的方法。我们将两种独立建立的基于组织的方法联系在一起,用于确定结直肠癌患者的预后:肿瘤脂肪特征(TAF)和基质活性侵袭前区(SARIFA)。作为生物特征的 SARIFA 仅由人类观察,而 TAF 则是通过计算机算法识别的。我们检查了许多癌症切片中的 TAF,看它们是否与 SARIFA 相似。TAF通常与SARIFA相匹配,但并非总是如此。有趣的是,这些方法可用于预测患者的预后,并与肿瘤和脂肪细胞相互作用的特定基因表达有关。我们的研究表明,将计算机算法与人类评估组织样本的专业知识相结合,可以在患者样本中识别出有意义的特征,这可能有助于预测最佳治疗方案。Reitsam 等人评估了基于深度学习和人类观察者的肿瘤与脂肪细胞相互作用识别之间的重叠,认为这是结肠直肠癌的一种有希望的生物标记物。他们证明,将人工智能与人类病理专家相结合有利于识别临床相关的生物标记物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


