Photochemical tandem reaction of nitrogen containing heterocycles, bicyclo[1.1.1]pentane, and difluoroiodane(iii) reagents†
IF 2.7
3区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
A visible light-induced difluoroalkylation/heteroarylation of [1.1.1]propellane with nitrogen containing heterocycles and difluoroiodane(iii) reagents was achieved. Various heteroarenes and difluoroiodane(iii) reagents exhibited good compatibility, yielding the desired products in moderate to good yields. The accessibility of the reagents and the mild reaction conditions establish this method as an alternative and practical strategy for accessing diverse 1-difluoroalkyl-3-heteroaryl bicyclo[1.1.1]pentanes (BCPs).
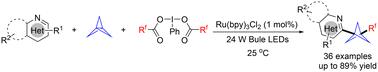
含氮杂环、双环[1.1.1]戊烷和二氟碘烷(III)试剂的光化学串联反应。
利用含氮杂环和二氟碘烷(III)试剂实现了可见光诱导的[1.1.1]丙烷二氟烷基化/异芳基化反应。各种杂环和二氟碘烷(III)试剂表现出良好的兼容性,以中等至良好的产率获得所需的产物。试剂的易获得性和温和的反应条件使该方法成为获得多种 1-二氟烷基-3-杂芳基双环[1.1.1]戊烷(BCPs)的一种替代性实用策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic & Biomolecular Chemistry
化学-有机化学
CiteScore
5.50
自引率
9.40%
发文量
1056
审稿时长
1.3 months
期刊介绍:
Organic & Biomolecular Chemistry is an international journal using integrated research in chemistry-organic chemistry. Founded in 2003 by the Royal Society of Chemistry, the journal is published in Semimonthly issues and has been indexed by SCIE, a leading international database. The journal focuses on the key research and cutting-edge progress in the field of chemistry-organic chemistry, publishes and reports the research results in this field in a timely manner, and is committed to becoming a window and platform for rapid academic exchanges among peers in this field. The journal's impact factor in 2023 is 2.9, and its CiteScore is 5.5.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


