Dynamic-to-static switch of hydrogen bonds induces a metal–insulator transition in an organic–inorganic superlattice
IF 19.2
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Hydrogen bonds profoundly influence the fundamental chemical, physical and biological properties of molecules and materials. Owing to their relatively weaker interactions compared to other chemical bonds, hydrogen bonds alone are generally insufficient to induce substantial changes in electrical properties, thus imposing severe constraints on their applications in related devices. Here we report a metal–insulator transition controlled by hydrogen bonds for an organic–inorganic (1,3-diaminopropane)0.5SnSe2 superlattice that exhibits a colossal on–off ratio of 107 in electrical resistivity. The key to inducing the transition is a change in the amino group’s hydrogen-bonding structure from dynamic to static. In the dynamic state, thermally activated free rotation continuously breaks and forms transient hydrogen bonds with adjacent Se anions. In the static state, the amino group forms three fixed-angle positions, each separated by 120°. Our findings contribute to the understanding of electrical phenomena in organic–inorganic hybrid materials and may be used for the design of future molecule-based electronic materials. Hydrogen bonds impact the chemical, physical and biological properties of molecular materials, but are rarely able to induce significant changes in electrical properties. Now a dynamic-to-static transition of hydrogen bonds in an organic–inorganic superlattice has been shown to yield a metal–insulator transition with an on–off ratio of 107 in electrical resistivity.
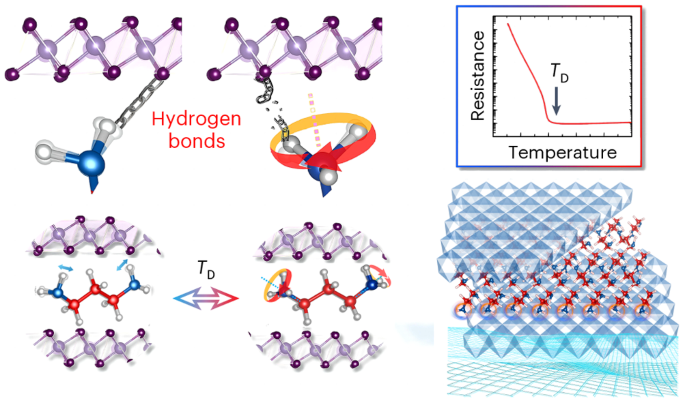
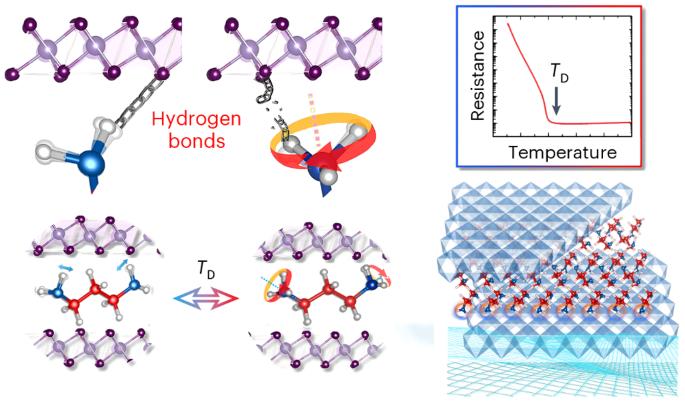
氢键的动静转换诱导有机-无机超晶格中的金属-绝缘体转变
氢键深刻影响着分子和材料的基本化学、物理和生物特性。与其他化学键相比,氢键的相互作用相对较弱,因此仅靠氢键一般不足以引起电学性质的实质性变化,从而严重制约了氢键在相关设备中的应用。在这里,我们报告了由氢键控制的有机-无机(1,3-二氨基丙烷)0.5SnSe2 超晶格的金属-绝缘体转变,其电阻率的开关比高达 107。诱导这种转变的关键在于氨基的氢键结构从动态转变为静态。在动态状态下,热激活的自由旋转不断断裂并与相邻的硒阴离子形成瞬时氢键。在静态下,氨基形成三个固定角度位置,每个位置之间相隔 120°。我们的发现有助于理解有机-无机杂化材料中的电现象,并可用于设计未来的分子电子材料。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature chemistry
化学-化学综合
CiteScore
29.60
自引率
1.40%
发文量
226
审稿时长
1.7 months
期刊介绍:
Nature Chemistry is a monthly journal that publishes groundbreaking and significant research in all areas of chemistry. It covers traditional subjects such as analytical, inorganic, organic, and physical chemistry, as well as a wide range of other topics including catalysis, computational and theoretical chemistry, and environmental chemistry.
The journal also features interdisciplinary research at the interface of chemistry with biology, materials science, nanotechnology, and physics. Manuscripts detailing such multidisciplinary work are encouraged, as long as the central theme pertains to chemistry.
Aside from primary research, Nature Chemistry publishes review articles, news and views, research highlights from other journals, commentaries, book reviews, correspondence, and analysis of the broader chemical landscape. It also addresses crucial issues related to education, funding, policy, intellectual property, and the societal impact of chemistry.
Nature Chemistry is dedicated to ensuring the highest standards of original research through a fair and rigorous review process. It offers authors maximum visibility for their papers, access to a broad readership, exceptional copy editing and production standards, rapid publication, and independence from academic societies and other vested interests.
Overall, Nature Chemistry aims to be the authoritative voice of the global chemical community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


