Radical strain-release photocatalysis for the synthesis of azetidines
IF 42.8
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
The increasing popularity of four-member rings in drug discovery has prompted the synthetic chemistry community to advance and reinvent old strategies to craft these structures. Recently, the strain-release concept has been used to build complex architectures. However, although there are many strategies for accessing small carbocyclic derivatives, the synthesis of azetidines remains underdeveloped. Here we report a photocatalytic radical strategy for accessing densely functionalized azetidines from azabicyclo[1.1.0]butanes. The protocol operates with an organic photosensitizer, which finely controls the key energy-transfer process with distinct types of sulfonyl imines. The radical intermediates are intercepted by the azabicyclo[1.1.0]butanes via a radical strain-release process, providing access to difunctionalized azetidines in a single step. This radical process is revealed by a combination of spectroscopic and optical techniques and density functional theory calculations. The power and generality of this method is illustrated with the synthesis of various azetidine targets, including derivatives of celecoxib and naproxen. Four-membered rings have become popular motifs in drug discovery, prompting the synthetic organic chemistry community to develop more expedite approaches to access these scaffolds. Here, the authors report a photocatalytic radical strategy for accessing densely functionalized azetidines from azabicyclo[1.1.0]butanes.
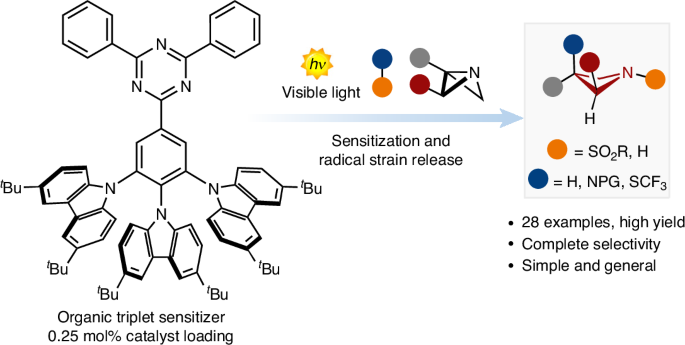
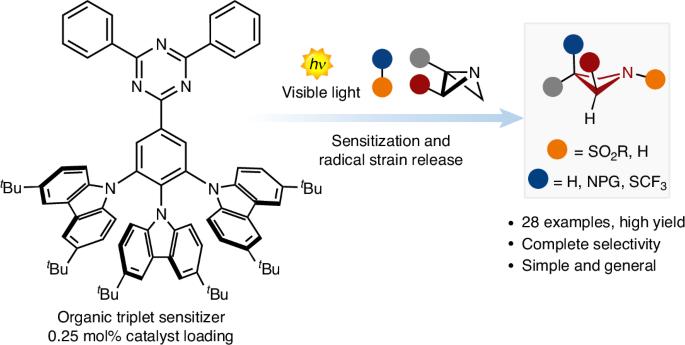
用于合成氮杂环丁烷的自由基应变释放光催化技术
四元环在药物研发中越来越受欢迎,这促使合成化学界不断改进和创新旧的策略,以打造这些结构。最近,应变释放概念被用于构建复杂的结构。然而,尽管有许多策略可以获得小型碳环衍生物,但氮杂环丁烷的合成仍然不够成熟。在此,我们报告了一种从氮杂双环[1.1.0]丁烷中获得致密官能化氮杂环丁烷的光催化自由基策略。该方案使用有机光敏剂,通过不同类型的磺酰亚胺精细控制关键的能量转移过程。氮杂双环[1.1.0]丁烷通过自由基应变释放过程拦截自由基中间体,从而在一个步骤中获得双官能化氮杂环丁烷。光谱和光学技术与密度泛函理论计算相结合,揭示了这一自由基过程。通过合成各种氮杂环丁烷目标物,包括塞来昔布和萘普生的衍生物,说明了这种方法的威力和通用性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Catalysis
Chemical Engineering-Bioengineering
CiteScore
52.10
自引率
1.10%
发文量
140
期刊介绍:
Nature Catalysis serves as a platform for researchers across chemistry and related fields, focusing on homogeneous catalysis, heterogeneous catalysis, and biocatalysts, encompassing both fundamental and applied studies. With a particular emphasis on advancing sustainable industries and processes, the journal provides comprehensive coverage of catalysis research, appealing to scientists, engineers, and researchers in academia and industry.
Maintaining the high standards of the Nature brand, Nature Catalysis boasts a dedicated team of professional editors, rigorous peer-review processes, and swift publication times, ensuring editorial independence and quality. The journal publishes work spanning heterogeneous catalysis, homogeneous catalysis, and biocatalysis, covering areas such as catalytic synthesis, mechanisms, characterization, computational studies, nanoparticle catalysis, electrocatalysis, photocatalysis, environmental catalysis, asymmetric catalysis, and various forms of organocatalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


